News Express: Joint study of UM and two universities of Chinese medicine helps alleviate sepsis-induced injury
新聞快訊:澳大與兩中醫藥大學聯合研究成果有效減緩敗血症

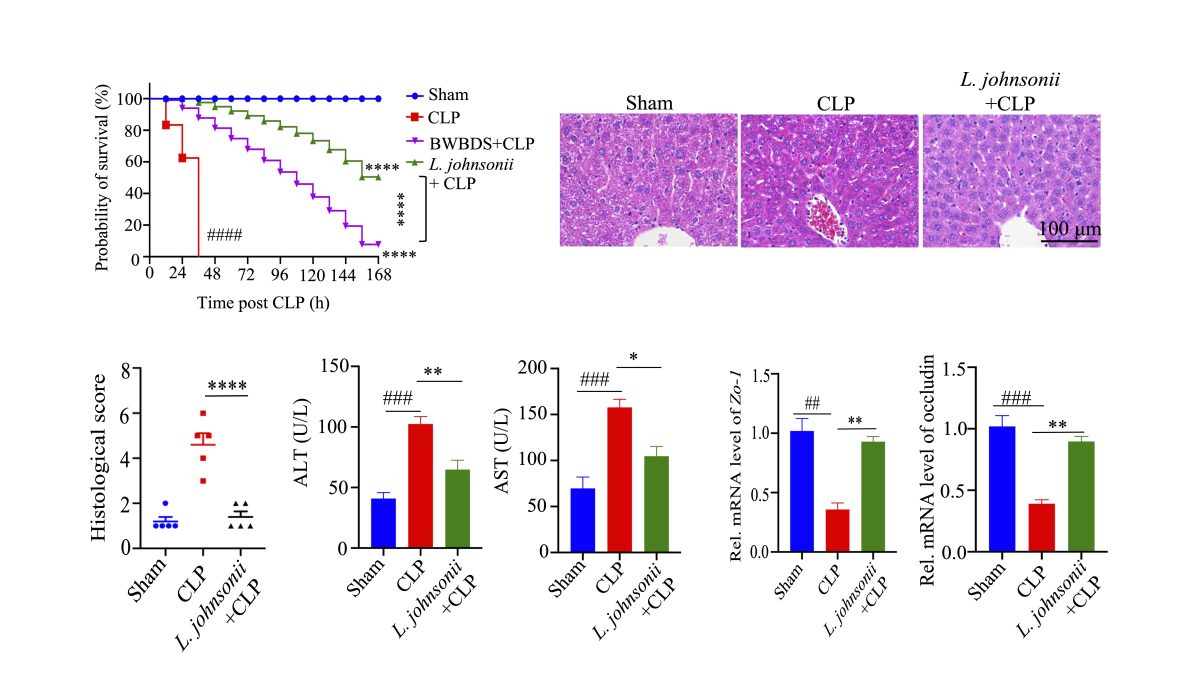
八味敗毒散可以有效延長CLP模型小鼠的生存期,緩解敗血症引起的肝損傷,增加約氏乳桿菌的豐度
BWBDS can effectively prolong the survival of CLP-treated mice, alleviate SILI, and promote the growth of L. johnsonii
澳大與兩中醫藥大學聯合研究成果有效減緩敗血症
澳門大學健康科學學院教授梁麗嫻、廣州中醫藥大學第二附屬醫院教授謝瑩及北京中醫藥大學教授陳建新共同帶領的團隊發現臨床現代經驗方“八味敗毒散(BWBDS)” 可以有效減緩敗血症和由其引發的肝損傷。相關研究成果在藥學領域備受矚目,並已獲國際知名藥學期刊《藥學學報》英文刊(Acta Pharmaceutica Sinica B)刊登。
敗血症被廣泛認為是一種全身器官功能障礙並危及生命的嚴重的感染性疾病,由宿主對感染的炎症反應引起。 抗生素是敗血症初期治療的有效藥物。然而,抗生素的不當使用可能會導致耐藥感染和死亡。因此,有必要探索新的靶向或聯合治療策略。腸—肝軸是指腸道和肝臟之間的微生物、代謝和免疫相互作用的雙向關係,通過門靜脈和膽道樹雙向連接。然而,膿毒症引起的肝損傷被認為是重症監護病房的一個關鍵問題。腸道菌群在膿毒症引起的肝損傷中起著至關重要的作用。中藥複方BWBDS是從八種傳統中藥(人參、百合、黃精、金銀花、沙棘、杏仁、桔梗和柏皮)提取的有效成分。研究團隊的前期研究表明藥食同源中藥配方能改善腸道微生態, 增強宿主自身機體免疫功能。在這項研究中,研究團隊旨在研究BWBDS治療是否可以通過調節腸道微生物群來逆轉敗血症引起的肝損傷。該研究表明八味敗毒散和約氏乳桿菌或將作為預防和治療敗血症的益生元和益生菌。
儘管BWBDS在治療術後感染的臨床療效顯著,但其藥理作用機制尚不清楚。研究團隊對通過高效液相色譜—串聯質譜技術(LC/MS/MS)對BWBDS的成分進行定性和定量分析。應用網路藥理學來預測八味敗毒散的可能藥理作用機制。盲腸結紮和穿刺手術(CLP)用於誘導術後感染引起的敗毒症和相關損傷。糞便菌群移植和16S rRNA PacBio單分子即時(SMRT)測序用於驗證腸道微生物群的作用。通過代謝組學分析以表徵代謝譜。使用氯膦酸鹽脂質體和抗白細胞介素-10受體小鼠抗體(anti-IL-10R mAb)探索腸道菌群減輕膿毒症損傷的潛在機制。
研究團隊發現中藥複方八味敗毒散在 CLP的小鼠中選擇性地促進了乳桿菌的生長。糞菌移植(FMT)治療表明腸道細菌與敗血症相關,並且是八味敗毒散抗敗血症作用所必需的。用約氏乳桿菌治療可顯著預防小鼠敗血症和敗血症引起的肝損傷,並提高小鼠的存活率,這與增強腸道完整性、減少腸道和全身炎症有關。值得注意的是,研究團隊的研究結果表明,約氏乳桿菌減輕CLP誘導的死亡和肝損傷取決於促進IL-10+M2巨噬細胞的表達。進一步的機制表明,熱滅活的約氏乳桿菌可以促進巨噬細胞抗炎功能,從而減輕敗血症誘導的肝損傷。
研究團隊的發現為膿毒症引起的肝損傷提供了一種新的治療方法。在可預見的將來,腸道菌群狀態,如物種多樣性,將被用作預測中藥複方BWBDS治療膿毒症患者效果的生物標誌物。此外,BWBDS和腸道細菌約氏乳桿菌作為治療敗血症患者的新型益生元和益生菌,或將轉化為臨床應用。該研究揭示了中藥複方通過調控腸道菌群治療敗血症的巨大應用潛力。
梁麗嫻、謝瑩及陳建新為研究的共同通訊作者,澳門科技大學博士生范小青、麥楚填及北京中醫藥大學博士生左玲為共同第一作者。該項目由澳門科學技術發展基金(檔案編號:0096/2018/A3,0111/2020/A3,0056/2020/AMJ和2001/2020/ALC)和澳大(檔案編號:SRG2022-00020-FHS)資助,亦是國家中醫藥管理局2020青年岐黃學者支持的項目。研究文章及專題報導的完整版本可瀏覽https://doi.org/10.1016/j.apsb.2022.10.016。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/campus-news/detail/55015/
Joint study of UM and two universities of Chinese medicine helps alleviate sepsis-induced injury
A research team jointly led by Elaine Leung Lai Han, professor in the Faculty of Health Sciences (FHS) of the University of Macau (UM); Xie Ying, professor at the Second Affiliated Hospital of the Guangzhou University of Chinese Medicine; and Chen Jianxin, professor at the Beijing University of Chinese Medicine has discovered that the modern herbal formula BaWeiBaiDuSan (BWBDS) can help alleviate sepsis-induced liver injury (SILI). The study has received considerable attention in the field of pharmacology and the research results have been published in the internationally-renowned journal Acta Pharmaceutica Sinica B.
Sepsis is widely perceived as a life-threatening organ dysfunction caused by a dysregulated host response to infection. Antibiotics are an essential component of initial therapy in sepsis. However, the inappropriate use of antibiotics can lead to multidrug-resistant infections and death. It is necessary to discover novel targeted or combined therapeutic strategies. The gut-liver axis refers to the bidirectional relationship of microbiota, metabolic, and immune crosstalks between the gut and liver, connected in a bidirectional fashion by the portal vein and biliary tree. SILI is related to adverse clinical outcomes and gut microbiota plays a crucial role in SILI. The formula BWBDS is based on components extracted from eight medicinal herbs, namely Panax ginseng C. A. Meyer, Lilium brownii F. E. Brown ex Miellez var. viridulum Baker, Polygonatum sibiricum Delar. ex Redoute, Lonicera japonica Thunb., Hippophae rhamnoides Linn, Amygdalus Communis Vas, Platycodon grandiflorus (Jacq.) A. DC., and Cortex Phellodendri. Previous studies by the team have showed that traditional Chinese medicine (TCM) formulas with medicine food homology can improve intestinal microecology and enhance host immune function. Therefore, in the study, the team investigated whether the BWBDS treatment could reverse SILI by modulating the gut microbiota. The study shows that BWBDS and L. johnsonii may act as prebiotics and probiotics for the prevention and treatment of sepsis.
Although the clinical efficacy of BWBDS is significant, the pharmacological mechanism of BWBDS remains unclear. The team performed qualitative and quantitative analysis of BWBDS components with high performance liquid chromatography-tandem mass spectrometry (LC/MS/MS). Network pharmacology was applied to predict possible mechanisms of BWBDS. Cecal ligation and puncture (CLP) was employed to induce sepsis and related injuries caused by post-operative infection. Faecal microbiota transplantation (FMT) and 16S PacBio single-molecule real-time (SMRT) sequencing were used to validate the roles of gut microbiota. Metabolomics analysis was performed to characterise the metabolic profile. Clodronate liposome and anti-interleukin-10 receptor mouse antibody (anti-IL-10R mAb) were used to explore the potential mechanism of bacteria attenuating SILI.
In the study, BWBDS selectively promoted the growth of Lactobacillus in CLP-treated mice. Results of the FMT treatment show that gut bacteria are correlated with sepsis and are required for BWBDS antisepsis effects. The treatment with L. johnsonii significantly prevented mice from sepsis and SILI and improved the survival of mice, which is associated with enhanced gut integrity, as well as reduced intestinal and systemic inflammation. In particular, the research results suggest that the reduction of CLP-induced mortality and alleviation of liver injury by L. johnsonii depend on the promotion of the proportions of IL-10+M2 macrophages. Moreover, the study shows that heat inactivation L. johnsonii (HI-L. johnsonii) treatment can promote the anti-inflammatory activity of macrophages and alleviate SILI.
The team’s findings provide a novel therapeutic approach for SILI. In the foreseeable future, gut microbiota status, such as species diversity, will be used as a biomarker to predict the therapeutic effects of BWBDS in sepsis patients. In addition, BWBDS and the gut bacterium L. johnsonii can be translated to clinical use as novel prebiotics and probiotics for the treatment of sepsis patients. The study has revealed the great potential of TCM in treating sepsis by regulating gut microbiota.
Prof Leung, Prof Xie, and Prof Chen are the co-corresponding authors of the study. PhD students Fan Xiaoqing and Mai Chutian at the Macau University of Science and Technology and PhD student Zuo Ling at the Beijing University of Chinese Medicine are the co-first authors. The project was funded by the Science and Technology Development Fund, Macao SAR (File no: 0096/2018/A3, 0111/2020/A3, 0056/2020/AMJ and 2001/2020/ALC) and UM’s research fund (SRG2022-00020-FHS), and was supported by the 2020 Qi Huang Young Scholar programme of the National Administration of Traditional Chinese Medicine. The full version of the paper can be viewed at https://doi.org/10.1016/j.apsb.2022.10.016.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/campus-news/detail/55015/