News Express: UM develops new technique for prediabetes detection
新聞快訊:澳大開發創新檢測技術篩查早期糖尿病
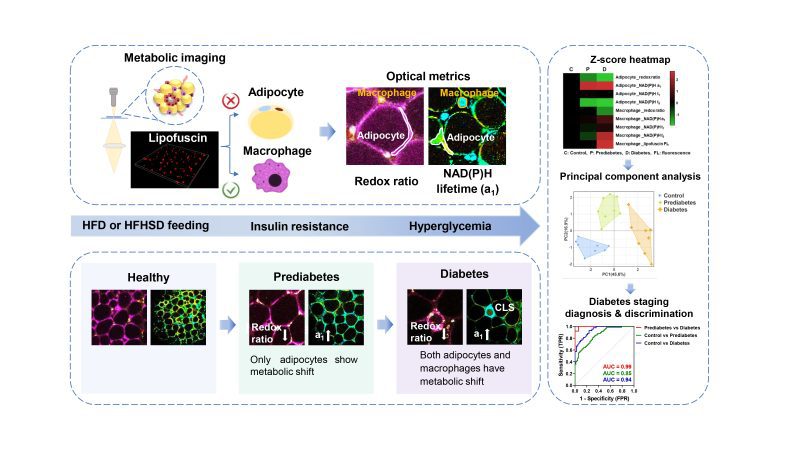
正常健康、早期糖尿病、晚期糖尿病的脂肪代謝螢光表徵可用於不同病理時期的鑑別診斷
The metabolic fluorescence features of healthy, prediabetic, and diabetic adipose tissues can be used for differential diagnosis of diabetes stages
澳大開發創新檢測技術篩查早期糖尿病
澳門大學健康科學學院副教授劉子銘帶領的團隊基於脂肪組織代謝螢光成像,開發早期糖尿病的檢測技術,揭示了新的糖尿病預防策略,並為診斷早期糖尿病提供了一種創新方法。相關研究成果已發表於國際期刊《治療診斷學雜誌》(Theranostics)。
早期糖尿病可以透過調整生活方式得以逆轉,但其主要病理特徵——胰島素抵抗卻無法像血糖測試一樣方便地檢測出來,糖尿病患者往往要等到出現高血糖時才被確診。因此,開發一種侵入性較低的診斷方法以快速評估胰島素抵抗,將有助於早期糖尿病的篩查預防。由於脂肪組織在早期糖尿病的發生和發展過程中起到關鍵作用,透過脂肪組織微環境的代謝螢光影像,表徵胰島素抵抗和炎症病理狀態,為診斷早期糖尿病提供了一種侵入性較低的檢查模式。
通過對三種內源性螢光團NAD(P)H、FAD、脂褐素的代謝成像,研究團隊成功發現早期糖尿病脂肪組織的可分化特徵。1040 納米激發的脂褐素自發螢光可標記出巨噬細胞的位置,這一特徵有助區分巨噬細胞的代謝螢光信號與脂肪細胞的代謝螢光信號。在有胰島素抵抗的早期糖尿病脂肪組織中,只有脂肪細胞表現出低氧化還原比的代謝螢光和高遊離NAD(P)H佔比a1。而對於從胰島素抵抗中恢復的小鼠,該差異性特徵消失了。當小鼠同時出現糖尿病性高血糖和脂肪組織發炎時,脂肪細胞和巨噬細胞都表現出相關代謝螢光變化。根據RNA-seq分析、生物能譜和組織病理學證據,脂肪細胞代謝螢光的變化可成為早期糖尿病胰島素抵抗的風險指標。
劉子銘為該研究的主要通訊作者,其博士生陳麗萍為第一作者。澳大健康科學學院博士生覃貴慧、博士後王曉燕等也對研究作出貢獻。該項目由澳門特別行政區科學技術發展基金(檔案編號:122/2016/A3, 018/2017/A1,0120/2020/A3, 0026/2021/A)和澳門大學(檔案編號:MYRG2018-00070-FHS, MYRG-CRG2022-00009-FHS)資助。全文可瀏覽:https://www.thno.org/v13p3550.htm。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/campus-news/detail/56379/
UM develops new technique for prediabetes detection
A research team led by Liu Tzu-Ming, associate professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), has developed a technique for prediabetes detection based on metabolic fluorescence imaging of adipose tissue. The study presents an innovative method for diagnosing prediabetes, shedding light on diabetes prevention strategies. The research results have been published in the international journal Theranostics.
Prediabetes can be reversed through lifestyle intervention, but its main pathologic hallmark, insulin resistance (IR), cannot be detected as conveniently as blood glucose testing. In consequence, the diagnosis of prediabetes is often delayed until patients have hyperglycemia. Therefore, developing a less invasive diagnostic method for rapid IR evaluation will contribute to the screening and prevention of prediabetes. Since adipose tissue is an endocrine organ that plays a crucial role in the development and progression of prediabetes, metabolic fluorescence imaging the prediabetic microenvironment of adipose tissues provides a less invasive alternative for the characterisation of IR and inflammatory pathology.
The research team successfully identified the differentiable features of prediabetic adipose tissues by employing the metabolic imaging of three endogenous fluorophores NAD(P)H, FAD, and lipofuscin pigments. 1040-nm excited lipofuscin autofluorescence could mark the location of macrophages. This unique feature helps distinguish the metabolic fluorescence signals of macrophages from those of adipocytes. In prediabetes fat tissues with IR, only adipocytes exhibit a low redox ratio of metabolic fluorescence and high free NAD(P)H fraction a1. This differential signature disappears in mice which quit the high-fat diet or high-fat-high-sucrose diet and recover from IR. When mice have diabetic hyperglycemia and inflamed fat tissues, both adipocytes and macrophages possess such kind of metabolic change. As confirmed with RNA-seq analysis and histopathology evidence, the change in adipocyte’s metabolic fluorescence could be an indicator or risk factor of prediabetic IR.
Prof Liu is the corresponding author of the study, and his PhD student Chen Liping is the first author. Others who have contributed to the study include PhD student Qin Guihui and postdoctoral fellow Wang Xiaoyan in the FHS. The project was supported by the Science and Technology Development Fund of the Macao SAR (File no: 122/2016/A3, 018/2017/A1, 0120/2020/A3, 0026/2021/A) and UM (File no: MYRG2018-00070-FHS, MYRG-CRG2022-00009-FHS). The full version of the research article can be viewed at https://www.thno.org/v13p3550.htm.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/campus-news/detail/56379/