News Express: UM achieves breakthrough in developing light-sensitive anti-cancer drugs
新聞快訊:澳大成功開發提高療效的光敏感抗癌藥
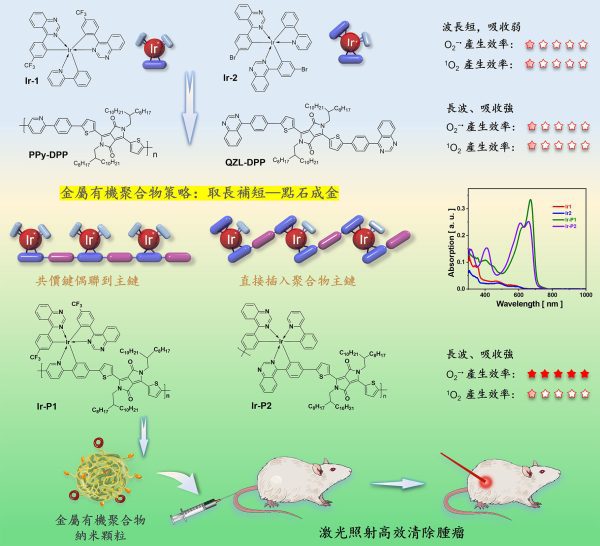
金屬聚合物策略開發高性能光敏抗癌藥物
A schema of the metallopolymerisation strategy for developing light-sensitive anti-cancer drugs
澳大成功開發提高療效的光敏感抗癌藥
澳門大學健康科學學院副教授張宣軍的研究團隊開發了高效光敏型抗癌藥物,不受氧氣濃度限制,有效解決光動力學治療過程中腫瘤微環境乏氧造成療效不佳的難題,在實用型光敏藥物開發方面獲得重大突破。該研究成果已於國際知名期刊《自然通訊》(Nature Communications)上刊登。
光動力學治療是化療、放療、手術治療和免疫治療以外的一種新型腫瘤治療方法,其原理是用光敏藥物在激光照射下通過光化學反應產生有毒的活性氧,高效殺死腫瘤細胞,具有低副作用、可協同治療等優點。根據產生活性氧物種的不同,光動力療法可以分為I型和II型,其中I型途徑是透過電子轉移生成超氧陰離子自由基、羥基自由基、過氧化氫等,而II型機制則透過能量傳遞將氧氣轉化為單線態氧。臨床上常規光動力學治療使用II型光敏劑,缺點是光化學過程高度依賴氧氣濃度,而腫瘤細胞透過高新陳代謝速率、高氧消耗等機制誘導腫瘤內部處於乏氧狀態,乏氧環境大大降低了光動力學治療的效果。因此,開發對氧氣依賴性低且吸收在長波段(對組織穿透力強)的I型光敏劑對臨床治療具有重要的意義。但由於缺乏有效通用性的設計策略,I型光敏劑的發展遠滯後於II型光敏劑。金屬銥配合物具有較強的自旋耦合作用和高的三線態激子產生效率,有望透過合理設計實現I型光化學反應。然而,其固有的短激發波長導致其有限的組織穿透度和嚴重的光損傷,成為限制它們在臨床應用上的主要障礙。
研究團隊把篩選出的銥配合物與半導體高分子材料經過合理的分子裁剪和組裝,成功開發了高效的光敏藥物,既能保持銥配合物的I型光化學,又充分利用半導體高分子在長波段強吸收的優勢。透過銥原子的點綴,半導體高分子產生活性氧的效率比原來提高了80倍。更重要的是,該策略普適性強,已成功拓展到一系列光敏藥物的開發。
目前,新開發的光敏藥物已成功用於小鼠模型乳腺癌的治療。該研究成果也以“金屬聚合物策略探索不受乏氧限制的窄帶隙光敏劑用於癌症的高效光動力治療”(Metallopolymer Strategy to Explore Hypoxic Active Narrow-Bandgap Photosensitizers for Effective Cancer Photodynamic Therapy)為題,發表於知名學術期刊《自然通訊》(Nature Communications)。
該研究的通訊作者為張宣軍,第一作者為澳大健康科學學院博士研究生張釗;澳大健康科學學院教授袁振、應用物理及材料工程研究院教授邢貴川和南方科技大學教授吳長鋒亦對研究作出重要貢獻。澳大健康科學學院動物研究核心實驗中心,蛋白質組學、代謝組學及藥物開發核心實驗中心、生物成像及幹細胞核心實驗中心為研究提供了優質服務。該項目獲澳門特別行政區科學技術發展基金(檔案編號:0085/2020/A2和0114/2019/A2)、廣東基礎和應用基礎研究基金(檔案編號:2022A151501061和2023A1515012524)、澳門大學(檔案編號:MYRG2020 -00130-FHS和MYRG2022-00036-FHS)、國家教育部澳門大學精準腫瘤學前沿科學中心的資助。全文可瀏覽:https://doi.org/10.1038/s41467-023-43890-z。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/campus-news/detail/57538/
UM achieves breakthrough in developing light-sensitive anti-cancer drugs
The research team led by Zhang Xuanjun, associate professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), has developed a highly effective photosensitive anticancer drug, representing a major breakthrough in the development of practical photosensitive drugs. The drug is not constrained by oxygen concentration and can effectively address the problem of low efficacy caused by hypoxia in the tumour microenvironment during photodynamic therapy. The research results have been published in the internationally renowned journal Nature Communications.
Photodynamic therapy (PDT) is a new type of tumour treatment in addition to chemotherapy, radiotherapy, surgical treatment, and immunotherapy. Its principle is to use photosensitive drugs to generate toxic reactive oxygen species (ROS) by photochemical reactions under laser irradiation, thereby effectively killing tumour cells. It has minimal side effects and is suitable for synergistic treatment. PDT can be divided into Type I and Type II depending on the different ROS produced. The Type-I pathway generates superoxide anion free radicals, hydroxyl radicals, and hydrogen peroxide by electron transfer, while the Type-II mechanism converts oxygen into singlet oxygen by energy transfer. In clinical practice, conventional PDT uses Type-II photosensitisers, which exhibit a disadvantage due to their high dependence on the concentration of oxygen during the photochemical process. The hypoxic microenvironment induced by mechanisms such as high metabolic rate and high oxygen consumption significantly diminishes the effectiveness of PDT. Therefore, the development of hypoxic active Type-I photosensitisers with low oxygen dependence and absorption in the long wavelength regions (deeper tissue penetration) is of great significance for clinical treatment. However, due to the lack of effective universal design strategies, the development of Type-I photosensitisers lags far behind that of Type-II photosensitisers. Organic iridium (III) complexes are a type of photosensitisers with excellent photocatalytic properties since the bountiful orbits of iridium atoms can promote the spin-orbit-coupling and lead to a higher probability of undergoing intersystem crossing, thus improving Type-I ROS generation. Despite this, their inherent short excitation wavelength results in limited tissue penetration and significant photodamage, which has become the main obstacle to their clinical application.
The research team successfully developed a highly effective photosensitive drug by tailoring the molecular structures of the selected iridium complexes and semiconducting polymers and assembling them. The drug can not only maintain the Type-I photochemistry of the iridium complexes, but also take full advantage of the strong absorption of semiconducting polymers in the long-wavelength region. With the incorporation of iridium atoms, the efficiency of metallopolymers in ROS generation is increased more than 80 times compared with the mother polymers. More importantly, this strategy is highly universal and has been successfully extended to the development of a series of photosensitive drugs.
The newly developed photosensitive drug has been successfully used in the treatment of breast cancer in mice models. The research results were published in Nature Communications under the title ‘Metallopolymer Strategy to Explore Hypoxic Active Narrow-Bandgap Photosensitizers for Effective Cancer Photodynamic Therapy’.
The corresponding author of the article is Zhang Xuanjun, and the first author is Zhang Zhao, a PhD graduate of FHS. Yuan Zhen, professor in FHS, Xing Guichuan, professor at the Institute of Applied Physics and Materials Engineering of UM, and Wu Changfeng, professor at Southern University of Science and Technology also made important contributions to the research. The Animal Research Core, the Proteomics, Metabolomics and Drug Development Core, and the Biological Imaging and Stem Cell Core provided high-quality services for the research. The project is supported by the Science and Technology Development Fund of the Macao SAR (File no: 0085/2020/A2 and 0114/2019/A2), the Guangdong Basic and Applied Basic Research Foundation (File no: 2022A151501061 and 2023A1515012524), UM (MYRG2020-00130-FHS and MYRG2022-00036-FHS), and the Ministry of Education Frontiers Science Center for Precision Oncology, UM. The full version of the research article can be viewed at https://www.nature.com/articles/s41467-023-43890-z.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/campus-news/detail/57538/