News Express: UM research team achieves breakthrough in pulmonary fibrosis treatment
新聞快訊:澳大研究團隊在肺纖維化治療取得突破
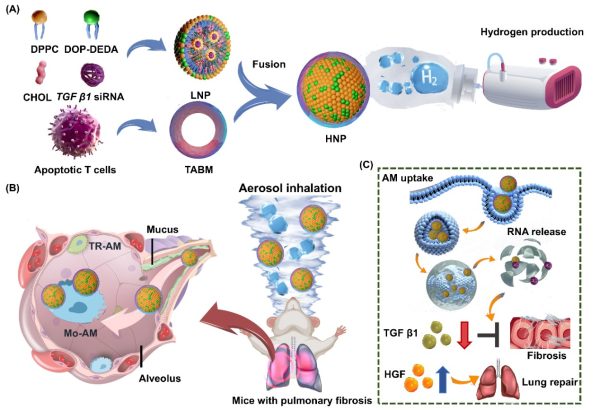
構建可吸入仿生HNP及自建氫氣驅動霧化給藥裝置用於治療肺纖維化
Schematic illustration of an inhalable biomimetic HNP delivered via a custom-designed aerosol inhalation device for the treatment of PF
澳大研究團隊在肺纖維化治療取得突破
澳門大學中華醫藥研究院教授鄭穎及健康科學學院副教授劉子銘的研究團隊成功研發出一種氫氣驅動的高效霧化吸入系統,能促進肺組織修復及有效阻斷促進肺部纖維化的信號通路,為臨床治療肺纖維化提供創新策略。該研究成果已刊登於國際頂級綜合性學術期刊《科學進展》(Science Advances)。
肺纖維化(PF)是一種慢性、進行性且致命的肺間質疾病,目前治療手段極為有限。近年來,肺部基因治療的進展顯示出阻止甚至逆轉肺纖維化的潛力,尤其是針對肺泡上皮細胞的基因突變校正。然而,儘管針對肺泡上皮細胞的基因療法前景可期,其臨床應用仍受制於肺部遞送系統的技術瓶頸。為突破這些限制,研究團隊開發了一種整合三大關鍵組件的霧化吸入系統:1、用於脂質納米顆粒(LNP)霧化的振動網式霧化器;2、利用氫氣的低黏度與抗炎特性增強黏液穿透能力;3、精準給藥控制艙以實現治療劑量的精確調控。研究假設氫氣的獨特性質(如降低雷諾數)可提升肺泡沉積效率並緩解氧化應激。
該策略未以傳統的上皮細胞為靶點,而是聚焦於促纖維化肺泡巨噬細胞(AMs)——此類細胞通過分泌TGFβ1驅動肺纖維化進程。研究團隊通過融合T細胞凋亡體膜與pH響應型DOP-DEDA脂質,設計出混合納米顆粒(HNPs),實現巨噬細胞特異性靶向並強化內體逃逸功能。這些HNPs可將TGFβ1 siRNA遞送至巨噬細胞,抑制纖維化信號傳導的同時,刺激肝細胞生長因子(HGF)生成以促進肺組織修復。氫氣霧化的HNPs在霧化過程中表現出優異穩定性,並通過層流輔助正壓穿透黏液屏障。該療法不僅阻斷纖維化通路,更重編程巨噬細胞表型,調控肺部微環境以實現持續療效。通過整合氫氣與生物膜雜化脂質納米粒,該研究提出一種雙重作用療法,同步解決肺纖維化的遞送挑戰與病理機制,為長期治療開闢新路徑。
該研究通訊作者為鄭穎,共同通訊作者為劉子銘,第一作者為澳大中華醫藥研究院博士生劉暢。研究由澳門特別行政區科學技術發展基金(檔案編號:005/2023/SKL、0086/2021/A2、0003/2023/RIC、0013/2023/RIC)、澳門大學(檔案編號:MYRG-GRG2023-00159-ICMS-UMDF、MYRG-CRG2022-00009-FHS)、國家藥品監督管理局藥用輔料質量控制與評價重點實驗室和廣東省藥品檢驗所(檔案編號:KF2022015)資助。全文可瀏覽:https://www.science.org/doi/10.1126/sciadv.adt2752。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/press-release/detail/61121/
UM research team achieves breakthrough in pulmonary fibrosis treatment
A research team led by Zheng Ying, professor in the Institute of Chinese Medical Sciences (ICMS) and Liu Tzu-Ming, associate professor in the Faculty of Health Sciences at the University of Macau (UM), has developed a highly efficient aerosol inhalation system. The system can promote lung tissue repair and effectively block fibrogenic signalling pathways, providing an innovative strategy for the clinical treatment of pulmonary fibrosis. The research has been published in the prestigious interdisciplinary journal Science Advances.
Pulmonary fibrosis (PF) is a chronic, progressive, and fatal interstitial lung disease with limited therapeutic options. Recent advancements in pulmonary gene therapy have shown the potential to halt or even reverse PF, particularly by focusing on correcting gene mutations in alveolar epithelial cells. Although gene therapies targeting alveolar epithelial cells have shown potential, their clinical application remains limited by technical bottlenecks in pulmonary delivery systems. To overcome these barriers, the research team developed an aerosol inhalation system that integrates three components: (1) a vibrating mesh nebuliser to aerosolise lipid nanoparticles (LNPs); (2) a hydrogen supplement system that exploits the low viscosity and anti-inflammatory properties of hydrogen to enhance mucus penetration; (3) a controlled dosing chamber to enable precise therapeutic delivery. The study hypothesised that hydrogen’s unique physicochemical properties (such as lower Reynolds numbers) could improve alveolar deposition and alleviate oxidative stress.
Rather than targeting epithelial cells, this strategy focused on profibrotic alveolar macrophages (AMs), which drive PF progression via TGFβ1 secretion. The team developed a hybrid lipid nanoparticle (HNP) by combining T cell apoptotic body membranes with pH-responsive DOP-DEDA lipids, allowing macrophage-specific targeting and enhanced endosomal escape. These HNPs delivered TGFβ1 siRNA to macrophages, suppressing fibrotic signalling while stimulating hepatocyte growth factor (HGF) production to promote lung tissue repair. The hydrogen-nebulised HNPs demonstrated stability during nebulisation and effectively penetrated the mucus barrier through laminar flow-assisted positive pressure. This approach not only blocks fibrotic pathways but also reprogrammes macrophage phenotypes, modulating the lung microenvironment to achieve sustained therapeutic effects. By integrating hydrogen gas with hybrid LNPs, the study presents a dual-action therapy that addresses both the delivery challenges and disease mechanisms of PF, offering a promising avenue for long-term treatment.
The corresponding author of the study is Prof Zheng Ying, with Prof Liu Tzu-Ming as co-corresponding author. The first author is Liu Chang, a PhD graduate in ICMS. The research was supported by the Science and Technology Development Fund of the Macao SAR (File No: 005/2023/SKL, 0086/2021/A2, 0003/2023/RIC, 0013/2023/RIC), UM (File No: MYRG-GRG2023-00159-ICMS-UMDF, MYRG-CRG2022-00009-FHS), NMPA Key Laboratory for Quality Control and Evaluation of Pharmaceutical Excipients and Guangdong Institute for Drug Control (File No: KF2022015). The full text of the study is available at: https://www.science.org/doi/10.1126/sciadv.adt2752.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/press-release/detail/61121/