News Express: UM research team develops pioneering biomimetic nanoantibiotics for treating bacterial pneumonia
新聞快訊:澳大團隊首創“仿生納米抗生素”治療細菌性肺炎
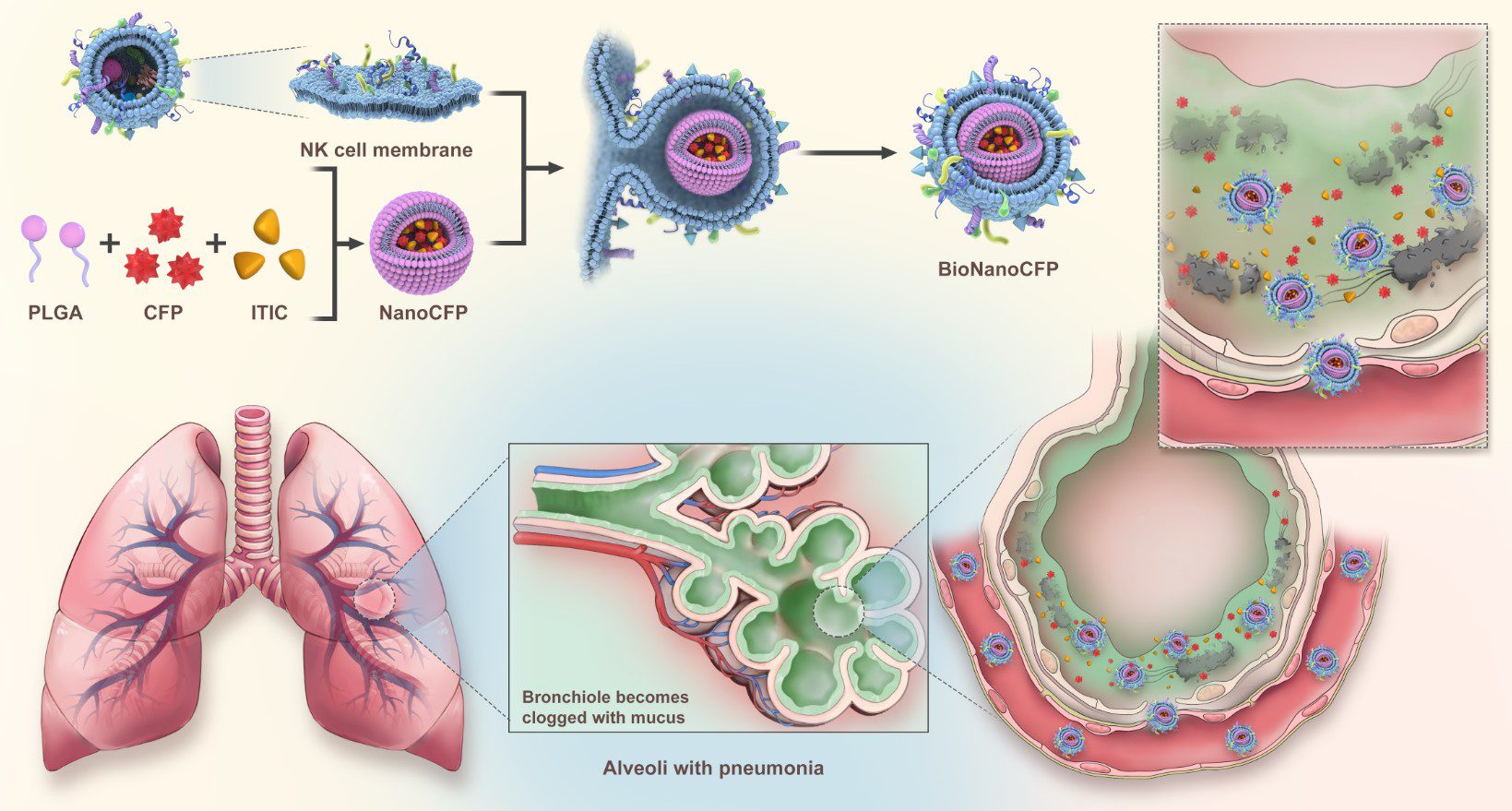
仿生抗生素治療的示意圖
A schematic of using biomimetic nanoantibiotics (BioNanoCFPs) for treating bacterial pneumonia
澳大團隊首創“仿生納米抗生素”治療細菌性肺炎
澳門大學健康科學學院副教授趙琦帶領的研究團隊攜手中國科學院科研力量,於細菌性肺炎治療領域取得革命性突破。團隊首創的“仿生納米抗生素”不僅能精準穿透肺部黏液屏障、高效遞送藥物至感染灶,更整合實時追蹤功能,為全球抗藥性細菌感染肺炎治療開闢全新路徑。該成果已刊登於國際頂級期刊《美國化學學會納米雜誌》,並獲iNature、納米前沿等知名學術媒體專題報導。
肺炎位列全球十大死因,每年奪走逾250萬人性命,其中細菌性肺炎因高併發症風險與逐年攀升的死亡率尤為棘手。儘管抗生素仍是治療主力,但其療效常受制於人體複雜的生理屏障:支氣管與肺部的緻密黏液層猶如“分子濾網”,阻擋藥物滲透並加速其清除,導致病灶區域藥物濃度不足,進而誘發細菌抗藥性。傳統靜脈注射的抗生素僅約2%能抵達感染部位,其餘大多在全身循環中代謝流失。這不僅降低療效,更增加肝腎毒性風險。突破黏液屏障、實現靶向遞送,是當代抗感染治療的關鍵挑戰。
澳大研究團隊從人體先天免疫系統獲取靈感,聚焦自然殺手(NK)細胞的獨特機能。此類免疫細胞表面富含Toll樣受體(TLR),可精準識別病原體分子標記,並穿透生物屏障直抵感染灶。團隊首創“NK細胞膜包覆納米載體”技術,將頭孢呱酮(Cefoperazone,CFP)抗生素與近紅外螢光分子吲啶二噻吩[3,2-b]噻吩(ITIC)封裝於仿生納米顆粒中,打造具備以下三個功能的治療平台:(1)智能導航:保留NK細胞膜表面的TLR與黏附分子,使載體能像天然免疫細胞般鎖定感染信號,穿越黏液層直達肺炎克雷伯菌聚集區;(2)實時追蹤:ITIC螢光標記賦予納米顆粒“可視化”特性,可透過影像技術監控藥物分佈,優化給藥策略;(3)定點爆破:在細菌富集區,載體受微酸環境觸發釋放CFP,局部藥物濃度較傳統給藥提升明顯,強效殺菌的同時避免全身毒性。
在重症肺炎小鼠模型中,單劑仿生納米抗生素治療即展現驚人效果,肺部細菌載量較傳統治療下降明顯,且無復發跡象。主要器官未見損傷,血液生化指標正常,證實其低毒性優勢。這項技術的突破性在於“仿生”與“智能化”的結合,不僅保留了原始細胞的抗原和表面結構,還賦予了納米平台多種生物學功能,如特異性配體識別、靶向疾病治療和穿越生物屏障的能力。仿生納米抗生素作為一種有效的細菌性肺炎治療方法,在未來的肺炎臨床治療中有巨大的應用潛力。
該研究通訊作者為趙琦、中國科學院深圳先進技術研究院研究員龔萍和張鵬飛、中國科學院上海藥物研究所研究員程震,第一作者為澳大健康科學學院博士畢業生王悅。該研究由澳門特別行政區科學技術發展基金(檔案編號:0009/2023/RIC和0043/2021/A1)和澳門大學(檔案編號:MYRG2022-00143-FHS和MYRG-GRG2023-00158-FHS-UMDF)資助。全文可瀏覽https://pubs.acs.org/doi/full/10.1021/acsnano.4c10837。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/press-release/detail/61370/
UM research team develops pioneering biomimetic nanoantibiotics for treating bacterial pneumonia
A research team led by Zhao Qi, associate professor in the Faculty of Health Sciences at the University of Macau (UM), has made a revolutionary breakthrough in the treatment of bacterial pneumonia. The team has developed pioneering biomimetic nanoantibiotics (BioNanoCFPs) that can penetrate the mucus barrier in the lungs for precise drug delivery to the infection site and enable real-time tracking. This innovation opens new avenues for treating antibiotic-resistant bacterial infections. The research has been published in the prestigious journal ACS Nano and covered by academic media platforms such as iNature and Nano Frontiers.
Pneumonia is one of the top ten causes of death worldwide, claiming over 2.5 million lives each year. Bacterial pneumonia, in particular, poses a significant challenge due to its high complication rate and increasing mortality rate. Although antibiotics remain the primary treatment for bacterial infections, their efficacy is often limited by the complex physiological barriers in the human body. The increased production of mucus in the bronchi and lungs forms a dense layer that obstructs drug penetration and accelerates drug clearance. This leads to an insufficient drug concentration at the infection site, which in turn promotes antibiotic resistance. Only about 2% of traditional intravenous antibiotics can reach the infection site, while the rest are metabolised and lost in the systemic circulation. This not only reduces treatment efficacy but also increases the risk of liver and kidney toxicity. Overcoming the mucus barrier to enable targeted drug delivery is a critical challenge in modern anti-inflammatory therapy.
Inspired by the innate immune system, the UM research team focused on the distinctive functions of natural killer (NK) cells. These immune cells are rich in Toll-like receptors (TLRs) on their surface, which enable them to recognise pathogen-associated molecular patterns within bacteria and penetrate biological barriers to reach the infection site. The team pioneered the technology for NK cell membrane-mediated drug delivery, and developed BioNanoCFPs by encapsulating cefoperazone (CFP) and indacenodithieno[3,2-b]thiophene (ITIC) in nanoparticles coated with NK cell membranes. These nanoplatforms have three key functions: (1) Smart navigation: TLRs and adhesion molecules on NK cell membranes are retained to enable the carriers to lock onto infection signals like natural immune cells, penetrate the mucus layer, and reach Klebsiella pneumoniae clusters; (2) Real-time tracking: fluorescence labelling of ITIC facilitates the visualisation of the targeting and treatment processes through imaging, thus optimising dosing; (3) Targeted release: the acidic microenvironment at bacterial colonisation sites triggers the release of CFP from the carriers, which significantly increases the local drug concentration compared to traditional drug delivery methods, achieving a potent antibacterial effect while avoiding systemic toxicity.
In mouse models of severe pneumonia, a single dose of BioNanoCFPs demonstrated remarkable efficacy, significantly reducing the bacterial load in the lungs compared to traditional treatments and showing no signs of recurrence. No damage to major organs was observed, and blood biochemical indicators remained normal, confirming the low toxicity of the treatment. The breakthrough lies in its combination of biomimetic and smart functions. It not only retains the antigens and surface structures of the original cells but also endows the nanoplatforms with several biological functions, including specific ligand recognition, targeted disease treatment, and the ability to penetrate biological barriers. With demonstrated efficacy against bacterial pneumonia, BioNanoCFPs hold enormous potential for future clinical applications.
The corresponding authors of the study are Prof Zhao; Gong Ping and Zhang Pengfei, researchers at the Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences; and Cheng Zhen, researcher at the Shanghai Institute of Materia Medica, Chinese Academy of Sciences. The first author is Wang Yue, a doctoral graduate of the Faculty of Health Sciences at UM. The research was funded by the Science and Technology Development Fund of the Macao SAR (File No.: 0009/2023/RIC and 0043/2021/A1), and UM (File No.: MYRG2022-00143-FHS and MYRG-GRG2023-00158-FHS-UMDF). The full version of the research article is available at: https://pubs.acs.org/doi/full/10.1021/acsnano.4c10837.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/press-release/detail/61370/