News Express: UM research team makes significant progress in study of drug resistance in non-small cell lung cancer
新聞快訊:澳大團隊在非小細胞肺癌耐藥性研究取得重要進展
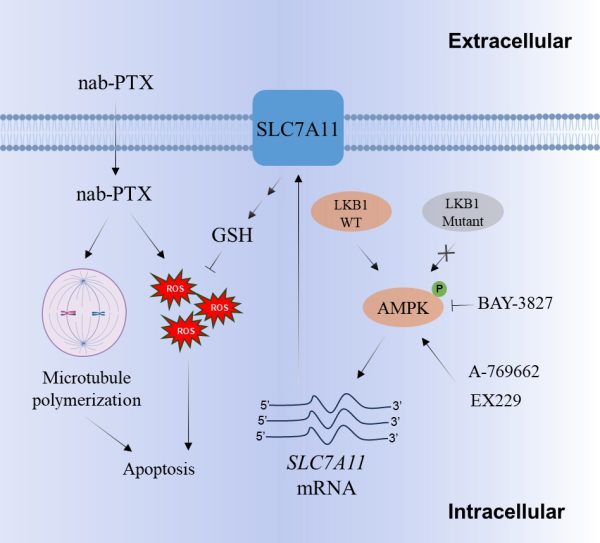
LKB1 減弱 nab-PTX 功效的機制及如何消除該問題的治療策略
The mechanism by which LKB1 reduces the therapeutic efficacy of nab-PTX and how this issue can be addressed
澳大團隊在非小細胞肺癌耐藥性研究取得重要進展
澳門大學健康科學學院講座教授沈漢明的研究團隊在非小細胞肺癌耐藥性的研究領域取得重要進展。該團隊揭示了部分患者對重要化療藥物白蛋白結合型紫杉醇產生耐藥性的分子機制,為開發克服此類耐藥性的治療策略提供了科學依據。相關成果已發表於國際知名期刊《氧化還原生物學》(Redox Biology)。
肺癌是發病率最高的癌症之一,且致死率在所有的惡性腫瘤中位居首位。非小細胞肺癌是肺癌的主要類型,佔據所有肺癌病例約85%。白蛋白結合型紫杉醇是治療晚期非小細胞肺癌的重要化療藥物,但其臨床療效受到腫瘤耐藥的極大限制。因此,闡明導致白蛋白結合型紫杉醇耐藥的機制並開發有效策略,對非小細胞肺癌患者至關重要。
澳大研究團隊通過一系列細胞生物學實驗和荷瘤小鼠模型,首次發現傳統被認為是腫瘤抑制因數的LKB1蛋白竟會促進非小細胞肺癌對白蛋白結合型紫杉醇的耐藥性。通過轉錄組測序分析,團隊發現在表達野生型LKB1蛋白的肺癌細胞中,AMPK信號通路顯著啟動。進一步實驗證實,LKB1通過啟動AMPK促進SLC7A11蛋白的表達,從而增加細胞內關鍵抗氧化劑谷胱甘肽(GSH)的含量。GSH可清除導致氧化應激和細胞死亡的活性氧(ROS),最終削弱白蛋白結合型紫杉醇的療效。研究團隊還發現,聯合使用AMPK抑制劑可顯著增強白蛋白結合型紫杉醇在體內外實驗中的抗腫瘤效果。這些發現為開發克服非小細胞肺癌對白蛋白結合型紫杉醇耐藥性的治療策略提供了新思路。
該研究第一作者為澳門大學健康科學學院博士生容達德,沈漢明、中山大學中山醫學院副教授盧廣、浙江省腫瘤醫院實驗研究中心主任凌志強等為研究作出重要貢獻。該研究獲澳門大學健康科學學院基因組學、生物信息學及單細胞分析核心實驗中心,生物影像及幹細胞核心實驗中心,動物研究核心實驗中心,澳門大學(檔案編號:CPG2023-00032-FHS、CPG2024-00035-FHS、MYRG2020-00022-FHS),澳門特別行政區科學技術發展基金(0078/2020/A2、0031/2021/A1、0081/2022/AMJ、0004/2021/AKP)等支持。全文可瀏覽:https://www.sciencedirect.com/science/article/pii/S2213231725000801。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/campus-news/detail/61409/
UM research team makes significant progress in study of drug resistance in non-small cell lung cancer
A research team led by Shen Hanming, chair professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), has made significant progress in the study of drug resistance in non-small cell lung cancer (NSCLC). The team has discovered the mechanisms behind some NSCLC patients’ resistance to albumin-bound paclitaxel (nab-PTX), an important chemotherapeutic drug. Their findings provide novel insights for the development of new therapeutic strategies to overcome such resistance. The research has been published in the international journal Redox Biology.
Lung cancer is one of the most frequently diagnosed cancers, and remains the leading cause of cancer-related death worldwide. NSCLC is the main type of lung cancer, accounting for about 85% of all cases. Nab-PTX is an important chemotherapy drug used to treat advanced NSCLC, but its clinical efficacy is greatly limited by drug resistance. Therefore, it is crucial to understand the underlying mechanisms and to develop effective strategies to overcome resistance and enhance the therapeutic efficacy of nab-PTX.
Through a series of cell biology experiments and xenograft mouse models, the research team made a groundbreaking discovery. They found that liver kinase B1 (LKB1), which is traditionally considered a tumour suppressor, contributes to nab-PTX resistance in NSCLC. Using RNA sequencing, the team discovered that the AMP-activated protein kinase (AMPK) signalling pathway was significantly upregulated in NSCLC cells with expression of wild-type LKB1. Subsequent experiments confirmed that LKB1 promotes the expression of SLC7A11 by activating AMPK, thereby increasing the intracellular level of glutathione (GSH), a key antioxidant. GSH eliminates the reactive oxygen species (ROS), which cause oxidative stress and cell death, ultimately weakening the therapeutic effect of LKB1. The team also discovered that combining nab-PTX with an AMPK inhibitor increases the therapeutic efficacy of nab-PTX in both in vitro and in vivo models. These findings provide new ideas for developing new therapeutic strategies to overcome nab-PTX resistance in NSCLC.
The first author of the study is Rong Dade, a doctoral student in FHS. Prof Shen; Lu Guang, associate professor in the Zhongshan School of Medicine at Sun Yat-sen University; Ling Zhiqiang, director of the Experimental Research Center at Zhejiang Cancer Hospital, also contributed to the study. The research was supported by the Genomics, Bioinformatics and Single Cell Analysis Core, the Biological Imaging and Stem Cell Core, and the Animal Research Core of FHS. It was also funded by UM (File No.: CPG2023-00032-FHS, CPG2024-00035-FHS, MYRG2020-00022-FHS), and the Science and Technology Development Fund of the Macao SAR (File No.: 0078/2020/A2, 0031/2021/A1, 0081/2022/AMJ, 0004/2021/AKP). The full version of the research article is available at: https://www.sciencedirect.com/science/article/pii/S2213231725000801.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/campus-news/detail/61409/