News Express: UM research team develops innovative scorpion venom drug delivery system to treat malignant brain tumours
新聞快訊:澳大團隊開發蠍毒攻克惡性腦瘤
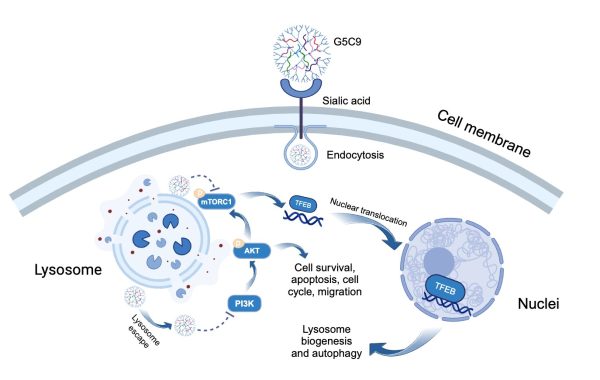
G5C9複合物通過胞吞作用進入細胞並殺死GBM細胞
Schematic representation of the presumed mechanism of action of G5C9 against glioblastoma cells
澳大團隊開發蠍毒攻克惡性腦瘤
由澳門大學健康科學學院教授郭珩輝領導的研究團隊,在高度侵襲性的腦腫瘤——膠質母細胞瘤(GBM)的靶向治療方面取得重大進展。該團隊創新性地將一種新型蠍源性十肽(Ctri9495, C9)與一種經過特異性修飾的樹枝狀納米載體(PAMAM dendrimer)相結合,成功構建了一種高效的藥物遞送系統,命名為G5C9。G5C9不僅能精確識別並有效穿透腫瘤細胞,還能通過激活自噬性細胞死亡通路顯著抑制腫瘤生長。該研究為克服GBM治療中的藥物遞送挑戰提供了一種有前景的策略,具有巨大的臨床轉化潛力,研究成果已發表於國際知名期刊《Journal of Controlled Release》。
膠質母細胞瘤是最常見且最具侵襲性的原發性中樞神經系統惡性腫瘤,其特點是患者預後極差,五年生存率僅約5%。現有的化療藥物,如替莫唑胺,常常因血腦屏障、血腦腫瘤屏障等障礙而難以有效到達腫瘤部位,同時腫瘤本身極易產生耐藥性。因此,迫切需要能夠有效穿透這些屏障、精確靶向腫瘤並有效殺死癌細胞的新型療法。
動物毒液肽以其結構多樣性和高生物活性而聞名,為開發新型抗癌藥物提供了寶貴的資源。然而,天然肽療法常常面臨諸如靶向性不足、體內穩定性差、細胞穿透困難等挑戰。為解決這些問題,郭珩輝及其研究團隊採用了創新的方法。首先,他們從西藏三脊豚蠍(Chaerilus tricostatus)的毒液中分離鑒定出一種新型疏水性十肽C9;為增強C9穿透細胞的能力,研究團隊選擇了第五代聚酰胺-胺樹枝狀大分子(G5 PAMAM)作為載體。為賦予腫瘤靶向能力,研究人員用4-(溴甲基)苯硼酸(PBA)修飾了載體表面,PBA能特異性識別並結合在GBM細胞表面過度表達的唾液酸(sialic acid),如同“智能導航系統”般發揮作用。最後,將C9肽通過化學方法偶聯到修飾後的樹枝狀大分子上,形成G5C9複合物。
G5C9複合物通過PBA與腫瘤細胞表面唾液酸的特異性結合實現高效靶向。與僅能結合在細胞膜上的游離C9肽不同,G5C9能被GBM細胞快速內吞,並主要定位於溶酶體內。G5C9在體內外均表現出強大的抗腫瘤活性,而游離的C9肽則無明顯效果。重要的是,該研究闡明G5C9獨特的作用機制:它下調了關鍵的促生存和增殖信號通路——PI3K/AKT/mTOR信號通路中關鍵蛋白的磷酸化水平。此外,G5C9破壞了溶酶體功能,並抑制了位於溶酶體膜上的mTOR複合物1(mTORC1)的活性,導致溶酶體生物發生和自噬的主要調控因子——轉錄因子EB(Transcription Factor EB, TFEB)發生去磷酸化並易位至細胞核。TFEB的核轉位激活了自噬溶酶體通路,具體表現為自噬體形成增加、自噬標誌物LC3-II水平升高以及自噬底物p62的降解,最終導致GBM細胞發生自噬性細胞死亡(autophagic cell death)。該策略不僅顯著增強了蠍毒肽C9的抗腫瘤功效,也為利用其他具有潛在活性但面臨遞送挑戰的生物活性肽治療腦部疾病和其他惡性腫瘤提供了新的技術平台和理論基礎。
該研究通訊作者為郭珩輝,第一作者為澳大健康科學學院原博士後研究員秦海心。澳大健康科學學院博士生羅思源、博士畢業生左偉敏,湖北工業大學教授曹志賤,以色列理工學院教授Yehuda G. Assaraf也對該研究做出重要貢獻。該研究得到澳大健康科學學院生物成像及幹細胞核心實驗中心,蛋白質組學、代謝組學及藥物開發核心實驗中心的關鍵支持。該項目由澳門特別行政區科學技術發展基金(檔案編號:0010/2021/AFJ和 0027/2022/A1)、2024年澳門大學—何鴻燊博士醫療拓展基金會“揚帆追夢、創啟未來”資助計劃(檔案編號:SHMDF-VSEP/2024/002)以及國家教育部澳門大學精準腫瘤學前沿科學中心(檔案編號:SP2025-00001-FSCPO)資助。全文可瀏覽: https://doi.org/10.1016/j.jconrel.2025.113780。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/campus-news/detail/61485/
UM research team develops innovative scorpion venom drug delivery system to treat malignant brain tumours
A research team led by Kwok Hang Fai, professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), has made significant progress in developing targeted therapies for glioblastoma (GBM), a highly aggressive brain cancer. By combining a novel scorpion venom-derived decapeptide (Ctri9495, C9) with a specifically modified dendritic nanocarrier (PAMAM dendrimer), the team has constructed an innovative and efficient nanoparticle drug delivery system, and named it G5C9. The G5C9 system is not only capable of precisely identifying and efficiently penetrating tumour cells but also can significantly inhibit tumour growth by activating autophagic cell death pathways. This research provides a promising strategy for addressing drug delivery challenges in GBM treatment, holding substantial potential for clinical translation. The findings have been published in the internationally renowned journal Journal of Controlled Release.
GBM is the most common and aggressive primary malignant tumour of the central nervous system, characterised by an extremely poor patient prognosis and a five-year survival rate of only about 5%. Existing chemotherapy drugs, such as temozolomide, often struggle to effectively reach the tumour site due to barriers such as the blood-brain barrier and the blood-brain tumour barrier, while the tumours themselves readily develop drug resistance. Therefore, there is an urgent need for novel therapies that can efficiently penetrate these barriers, precisely target the tumour, and effectively kill cancer cells.
Animal venom peptides, known for their structural diversity and high bioactivity, offer a valuable resource for developing new anti-cancer drugs. However, natural peptide therapeutics often encounter challenges such as insufficient targeting, poor stability in vivo, and difficulties in cell penetration. To address these issues, Prof Kwok and his research team adopted an innovative approach. First, they identified a novel hydrophobic decapeptide, C9, in the venom of the scorpion Chaerilus tricostatus. Then, to enhance C9’s ability to penetrate cells, the team selected a fifth-generation polyamidoamine dendrimer (G5 PAMAM) as the carrier. To add tumour-targeting ability, the team modified the carrier’s surface with 4-(bromomethyl) phenylboronic acid (PBA), which specifically detects and binds to sialic acid that is overexpressed on GBM cell surfaces, acting like an ‘intelligent navigation system’. Finally, the C9 peptide was chemically conjugated to the modified dendrimer to form the G5C9 complex.
The G5C9 complex achieves efficient targeting through the specific binding of PBA to sialic acid on the tumour cell surface. Unlike the free C9 peptide, which only binds to the cell membrane, G5C9 is rapidly endocytosed by GBM cells and primarily localises within lysosomes. G5C9 demonstrates potent anti-tumour activity both in vitro and in vivo, while free C9 peptide shows no significant effect. Importantly, the study has elucidated G5C9’s unique mechanism of action: it downregulates the phosphorylation levels of key proteins in the crucial pro-survival and proliferation PI3K/AKT/mTOR signalling pathway. Furthermore, G5C9 disrupts lysosomal function and inhibits the activity of mTOR complex 1 (mTORC1) located on the lysosomal membrane. This disruption leads to the dephosphorylation and nuclear translocation of Transcription Factor EB (TFEB), the master regulator of lysosomal biogenesis and autophagy. TFEB’s nuclear translocation activates the autophagosome-lysosome pathway (ALP), as evidenced by increased autophagosome formation, elevated levels of the autophagy marker LC3-II, and degradation of the autophagy substrate p62, ultimately resulting in autophagic cell death in GBM cells. This strategy not only significantly enhances the anti-tumour efficacy of the scorpion venom peptide C9 but also provides a new technological platform and theoretical foundation for utilising other bioactive peptides that have potential activity but face delivery challenges in treating brain diseases and other malignancies.
The corresponding author of the study is Prof Kwok. The first author is Qin Haixin, a former postdoctoral fellow in FHS. Significant contributions were also made by Luo Siyuan, a doctoral student in FHS; Zuo Weimin, a doctoral graduate from FHS; Cao Zhijian, professor at Hubei University of Technology; and Yehuda G. Assaraf, professor at the Technion-Israel Institute of Technology and the recipient of 2024 UM Distinguished Visiting Scholar in FHS. The study received crucial support from the Biological Imaging and Stem Cell Core, as well as the Proteomics, Metabolomics and Drug Development Core in FHS. The research project was funded by the Science and Technology Development Fund of the Macao SAR (File No.: 0010/2021/AFJ and 0027/2022/A1), the UM–Dr. Stanley Ho Medical Development Foundation ‘Set Sail for New Horizons, Create the Future’ Grant 2024 (File No.: SHMDF-VSEP/2024/002), and the Ministry of Education Frontiers Science Center for Precision Oncology, University of Macau (File No.: SP2025-00001-FSCPO). The full version of the research article is available at: https://doi.org/10.1016/j.jconrel.2025.113780.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/campus-news/detail/61485/