News Express: UM uncovers key role of proteasome in overcoming cancer drug resistance
新聞快訊:澳大團隊揭蛋白酶體在癌細胞抗藥的關鍵作用
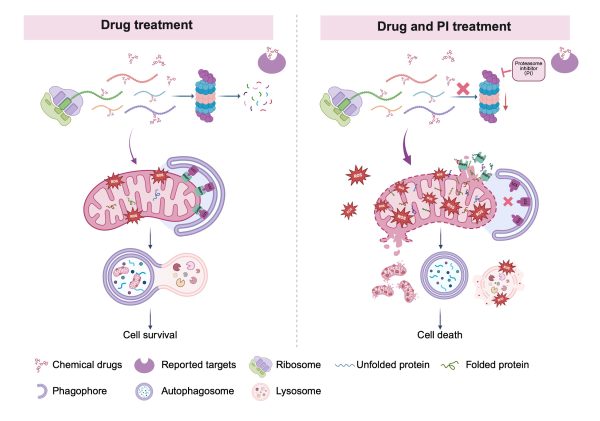
蛋白酶體抑制線粒體蛋白質輸入及促進ROS-BNIP3介導線粒體自噬
Proteasomes inhibit mitochondrial protein import and promote ROS-BNIP3-mediated mitophagy
澳大團隊揭蛋白酶體在癌細胞抗藥的關鍵作用
澳門大學健康科學學院講座教授鄧初夏領導的研究團隊在癌細胞抗藥性機制研究方面取得突破性進展。研究指出,蛋白酶體參與調控由抗癌藥物誘導的ROS-BNIP3介導線粒體自噬過程,進而影響癌細胞的存活與抗藥性。該成果為克服癌症抗藥性提供新線索,並已發表於國際權威期刊《Drug Resistance Updates》。
腫瘤細胞的多重抗藥性與蛋白酶體活性增強和線粒體功能下降密切相關。蛋白酶體的活性有助於清除受損蛋白質,而線粒體功能降低則可能導致細胞進入靜止狀態。然而,蛋白質損傷、線粒體功能和蛋白酶體活性之間的機制聯繫仍未明確。澳大研究團隊利用螢光藥物、可誘導線粒體蛋白模型和質譜技術,探索相關因素在腫瘤多重抗藥性中的潛在關聯。
研究主要發現包括:一、化學藥物與新合成的線粒體蛋白質結合,並共同輸入線粒體;二、輸入線粒體的受損蛋白質會觸發線粒體—溶酶體介導的鏈式反應,包括整合壓力反應(ISR)和線粒體非折疊蛋白反應(UPRmt),隨後導致溶酶體生物合成增加及ROS-BNIP3介導的線粒體自噬;三、蛋白酶體透過監測蛋白質穩態、抑制線粒體蛋白質輸入和促進正常、藥物處理條件下的線粒體自噬,使癌細胞能對抗藥物處理而存活;四、蛋白酶體抑制劑硼替佐米(BTZ)可引發線粒體過度輸入受損蛋白質,造成線粒體膜損傷,嚴重的線粒體ROS產生和洩漏、溶酶體膜通透性增加,使線粒體自噬無法產生;五、BTZ+藥物聯合治療可透過抑制線粒體自噬而誘導一種新型的細胞死亡,從而克服腫瘤抗藥性。
該研究通訊作者為鄧初夏及澳大健康科學學院客席副教授徐曉玲,第一作者及共同第一作者分別為該學院博士生李玲、博士後馮楊洋。其他團隊成員包括該學院研究助理教授邵方元、孫恆,博士生唐東洋、林詩琪、褚祥鵬、喬雲峰、李杭杭,博士後周靖波、雷海鵬。該研究獲國家自然科學基金(檔案編號:82030094)、澳門特別行政區科學技術發展基金(檔案編號:0004/2021/AKP、0007/2021/AKP、0129/2024/RIA2、0054/2023/RIA1、0065/2021/A、0193/2024/AGJ和0009/2022/AKP)和澳大(檔案編號:CPG2025-00035-FHS、MYRG2022-00175-FHS、MYRG-GRG2023-00029-FHS-UMDF和MYRG-GRG2024-00073-FHS)資助。全文可瀏覽:https://www.sciencedirect.com/science/article/pii/S1368764625000974。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/campus-news/detail/62556/
UM uncovers key role of proteasome in overcoming cancer drug resistance
A research team led by Chuxia Deng, chair professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), has made a significant breakthrough in understanding the mechanisms of cancer drug resistance. The study reveals that proteasomes play a key role in controlling ROS-BNIP3-mediated mitophagy triggered by cancer drugs, and this process impacts the survival and drug resistance of cancer cells. This finding offers new insights into how to overcome cancer drug resistance. The research has been published in the internationally renowned journal Drug Resistance Updates.
Multidrug resistance (MDR) is associated with increased proteasome activity, which facilitates the clearance of damaged proteins, and reduced mitochondrial activity, which contributes to quiescence. However, the mechanistic link between protein damage, mitochondrial dysfunction, and proteasome activity remains elusive. To explore the potential link between these factors in MDR, the research team utilised fluorescent drugs, inducible mitochondrial protein models, and mass spectrometry.
The key findings of the study are as follows: 1. Chemical drugs bind to newly synthesised mitochondrial proteins, and are imported into the mitochondria. 2. Damaged proteins that are imported into the mitochondria trigger a mitochondrion-lysosome-mediated chain reaction, including the integrity stress response (ISR) and the mitochondrial unfolded protein response (UPRmt), followed by increased lysosome biogenesis and ROS-BNIP3-mediated mitophagy. 3. The proteasome monitors proteostasis, suppresses mitochondrial protein import, and promotes mitophagy under both normal and drug-treated conditions. 4. The proteasome inhibitor bortezomib (BTZ) can trigger the excessive mitochondrial import of damaged proteins, causing mitochondrial membrane damage, profound mitochondrial ROS production and leakage, lysosome membrane permeabilisation, and impaired mitophagy. 5. Combining BTZ with other drugs can induce a new type of cell death by inhibiting mitochondrial autophagy, thereby overcoming tumour drug resistance.
Prof Deng and Xu Xiaoling, adjunct associate professor in FHS, are the corresponding authors of the study. Li Ling, a doctoral student in FHS, is the first author, and Feng Yangyang, a postdoctoral fellow in FHS, is the co-first author. FHS members who also contributed to the study include Research Assistant Professors Shao Fangyuan and Sun Heng; doctoral students Tang Dongyang, Lin Shiqi, Chu Xiangpeng, Qiao Yunfeng, and Li Hanghang; and post-doctoral fellows Zhou Jingbo and Lei Haipeng. The research project was funded by the National Natural Science Foundation of China (NSFC) (File No.: 82030094), the Science and Technology Development Fund of the Macao SAR (File Nos.: 0004/2021/AKP, 0007/2021/AKP, 0129/2024/RIA2, 0054/2023/RIA1, 0065/2021/A, 0193/2024/AGJ and 0009/2022/AKP), and UM (File Nos.: CPG2025-00035-FHS, MYRG2022-00175-FHS, MYRG-GRG2023-00029-FHS-UMDF, and MYRG-GRG2024-00073-FHS). The full version of the research article can be accessed at: https://www.sciencedirect.com/science/article/pii/S1368764625000974.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/campus-news/detail/62556/