News Express: UM develops novel antibacterial drug against drug-resistant bacteria
新聞快訊:澳大發現新型藥物對抗耐藥細菌
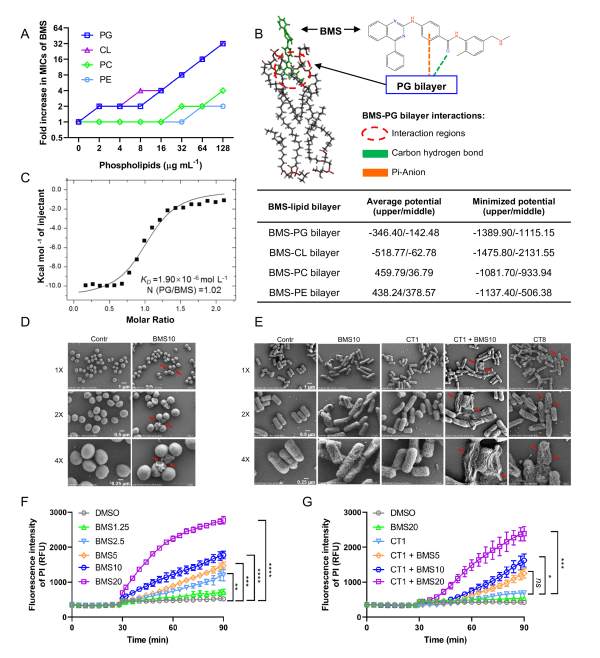
新型抗菌化合物BMS特異性地靶標細菌細胞膜磷脂
The novel antibacterial compound BMS specifically targets phospholipids in the bacterial membrane
澳大發現新型藥物對抗耐藥細菌
澳門大學健康科學學院副教授李子安的研究團隊鑒定了一種新型抗菌藥物,可以有效殺滅抗生素治療無效的耐藥細菌,為治療耐藥細菌感染找到新策略,在細菌研究上取得重大進展。該研究目前已發表於國際知名學術期刊《國際抗菌劑雜誌》(International Journal of Antimicrobial Agents)。
細菌感染曾是對人類健康威脅最大的疾病之一。中世紀的歐洲曾爆發過人類歷史最嚴重的瘟疫——“黑死病”,在全球造成約2億人死亡,是人類歷史上致死人數最多的流行病。後經證實,導致“黑死病”的元兇就是被稱為鼠疫桿菌的一種細菌。過去,由於缺乏有效的治療手段,哪怕是一個小小的傷口發生細菌感染,都有可能危及生命。這種黑暗時刻隨著抗生素的出現迎來了曙光:抗生素使人們能有效治療創傷感染、呼吸道感染以及安全地進行各種手術。然而,隨著抗生素的大量使用,細菌逐漸進化出逃逸抗生素殺傷的能力,即抗生素耐藥性。隨著越來越多的細菌被發現能抵抗多種甚至所有臨床抗生素的殺傷,抗生素發現前的黑暗時刻似乎又將再次來臨。因此,開發新型抗菌藥物以解決目前日益嚴峻的抗生素耐藥性問題已經迫在眉睫。
為快速找到有效對抗耐藥細菌的新型藥物,澳大研究團隊對一個商業化的化合物文庫進行篩選。該文庫中的大多數化合物都是已被批准治療非細菌感染的藥物,重新利用這些獲批藥物來治療耐藥細菌感染,不僅能大幅縮減新藥研發的時間,還能避免一些未知的副作用,充分開發這些藥物的臨床價值。經過一系列的篩選工作,研究團隊發現了一種被稱為BMS的化合物。該化合物不僅可以直接殺傷耐藥細菌,還可以增強臨床抗生素粘菌素的治療效果。經過測試,該化合物對臨床上許多耐藥細菌甚至多重耐藥細菌均有較好的治療作用。另外,研究團隊還對該化合物在細菌上的作用靶點以及它的作用機制進行解析。研究發現,該化合物主要是以細菌細胞膜上的磷脂成分作為作用靶點,並通過破壞細菌細胞膜及導致細菌代謝紊亂來發揮抗菌作用。研究團隊的研究工作為治療耐藥細菌感染及開發新的抗菌藥物以對抗抗生素耐藥性危機提供了新的思路。
李子安及澳大健康科學學院前副教授鄭軍為該研究的通訊作者,博士研究生張年為第一作者。該學院副教授譚建業、博士生單文穎、高亮亮及高詩鎧,北京大學深圳醫院技術人員盧暢及楊會林,中山大學教授彭博等均對該研究作出重要貢獻。該研究由澳門特別行政區科學技術發展基金(檔案編號:0016/2020/A1)及澳門大學(檔案編號:MYRG2020-00226-FHS和MYRG2022-00240-FHS)資助。全文可瀏覽:https://pubmed.ncbi.nlm.nih.gov/37328075/。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/campus-news/detail/56239/
UM develops novel antibacterial drug against drug-resistant bacteria
A research team led by Lee Tsz On, associate professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), has made a breakthrough in the study of bacteria. They have identified a novel antibacterial compound that can effectively kill bacterial pathogens that are resistant to antibiotic treatments, providing a new strategy for treating drug-resistant bacterial infections. The research results have been published in the internationally renowned journal International Journal of Antimicrobial Agents.
Bacterial infections were once one of the greatest threats to human health. The deadliest pandemic in human history, known as the Black Death, devastated medieval Europe, resulting in approximately 200 million deaths worldwide. It was later confirmed that the culprit behind the Black Death was the bacterium Yersinia pestis. In the past, due to the lack of effective treatment methods, even a minor bacterial infection in a wound could be life-threatening. With the advent of antibiotics, this dark period saw a ray of hope. Antibiotics enabled us to treat wound infections and respiratory tract infections effectively, as well as perform various surgeries safely. However, with the widespread use of antibiotics, bacteria have gradually evolved the ability to evade antibiotic killing, leading to antibiotic resistance. As more and more bacteria are proving resistant to multiple or even all clinically available antibiotics, it seems that another dark period similar to the pre-antibiotic era is upon us. Therefore, the development of new antibacterial drugs has become imminent to address the growing problem of antibiotic resistance.
To quickly identify new drugs that can effectively combat drug-resistant bacteria, the research team screened a commercial chemical compound library. Most of the compounds in the library are already approved for treating non-bacterial infections. Reutilising these approved drugs to treat drug-resistant bacterial infections would not only significantly reduce the time required for new drug development, but also avoid potential unknown side effects and fully explore the clinical value of these drugs. After a series of screenings, the research team discovered a compound called BMS. This compound can not only kill drug-resistant bacteria directly, but also enhance the therapeutic effects of the clinical antibiotic colistin. The results showed that the compound exhibits good therapeutic effects against many drug-resistant clinical bacteria and even multidrug-resistant bacteria. In addition, the research team also analysed the BMS compound’s target on bacteria and its mechanism of action. The study found that the compound primarily targets the phospholipids on the bacterial cell membrane and exerts its antibacterial effect by damaging the bacterial cell membrane and causing metabolic disturbances in the bacteria. The research provides new insights into the treatment of drug-resistant bacterial infections and the development of novel antibacterial drugs to combat the antibiotic resistance crisis.
Prof Lee and Zheng Jun, former associate professor in the FHS, are the corresponding authors of the study. PhD student Zhang Nian is the first author. Associate Professor Tam Kin Yip, and PhD students Shan Wenying, Gao Liangliang, and Kou Si Hoi in the FHS, technicians Lu Chang and Yang Huilin from Peking University Shenzhen Hospital, and Prof Peng Bo from Sun Yat-sen University also made important contributions to the study. The research was supported by the Science and Technology Development Fund of the Macao SAR (File no: 0016/2020/A1), and UM (File no: MYRG2020-00226-FHS and MYRG2022-00240-FHS). The full version of the paper can be viewed at https://pubmed.ncbi.nlm.nih.gov/37328075/.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/campus-news/detail/56239/