News Express: UM develops novel nanomedicine for augmenting cancer immunotherapy
新聞快訊:澳大新型納米藥提高治療腫瘤效果
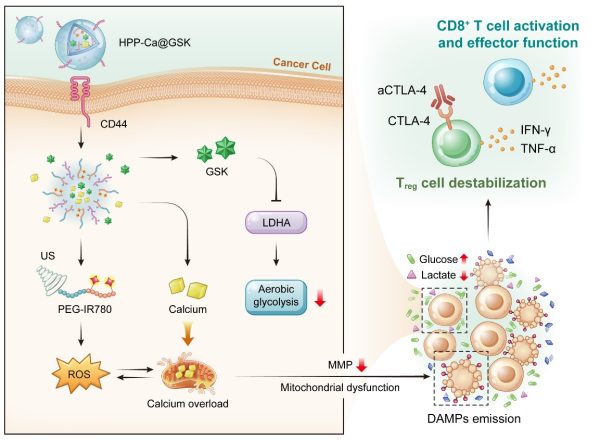
HPP-Ca@GSK納米藥物通過聲-代謝療法提升T細胞抗腫瘤免疫效應
A schematic illustration of HPP-Ca@GSK nanomedicines for augmenting T-cell antitumour immunity via sono-metabolic therapy
澳大新型納米藥提高治療腫瘤效果
澳門大學健康科學學院副教授代雲路帶領的團隊在腫瘤免疫治療領域取得了重要的研究進展。該研究構建一種新型金屬—多酚納米藥物,通過結合超聲介導的氧化應激和有氧糖酵解抑製劑來調控腫瘤細胞的代謝功能。這種“聲—代謝”療法有助於重新編程腫瘤微環境,從而提高T細胞的抗腫瘤免疫效應。相關研究成果已發表在材料領域知名期刊《美國化學會—納米》(ACS Nano)上。
腫瘤細胞和免疫細胞的代謝功能是影響其活力和功能的關鍵因素。惡性腫瘤的一個重要特徵是,即使在氧氣充足的條件下,腫瘤細胞也會通過糖酵解分解葡萄糖產生乳酸,這種現象被稱為“有氧糖酵解”。研究發現,腫瘤微環境中的具有高代謝活性的腫瘤細胞會與免疫細胞對周圍有限的營養物質進行爭奪。腫瘤細胞的這種高葡萄糖攝取和乳酸產生的能力,不僅剝奪了CD8+ T細胞的能量來源,抑制其效應功能,而且有助於調節性T細胞保持高免疫抑制能力。
基於此,該團隊開發了一種新型金屬—多酚納米藥物HPP-Ca@GSK,通過聲—代謝療法來誘導線粒體功能障礙和腫瘤微環境重編程。具體而言,團隊引入了LDHA抑製劑GSK2837808A來抑制腫瘤細胞有氧糖酵解,重建高葡萄糖、低乳酸的腫瘤微環境,這有利於提高CD8+ T細胞的效應功能和調節性T細胞的不穩定性。此外,超聲介導的氧化應激促使線粒體鈣離子過載,放大了線粒體功能障礙,從而促進損傷相關的分子模式釋放。這有助於促進樹突狀細胞的成熟和CD8+ T細胞的激活,使得更多活化的CD8+ T細胞浸潤到腫瘤部位。最後,與aCTLA-4抗體聯用會進一步增強調節性T細胞的不穩定性,提高聲—代謝腫瘤治療的療效。
該研究的通訊作者為代雲路和澳門大學健康科學學院研究助理教授李蓓,共同第一作者為學院博士生顏潔、研究助理李文曦和博士生田浩。博士生余馨穎、博士畢業生王國浩和桑瑋也對該研究做出重要貢獻。健康科學學院蛋白質組學、代謝組學及藥物開發核心實驗中心、動物研究核心實驗中心和生物成像及幹細胞核心實驗中心亦為研究提供了大力支持。此項研究由國家自然科學基金(檔案編號:32222090、32171318和32101069)、澳門大學(檔案編號:MYRG2022-00011-FHS)、澳門特別行政區科學技術發展基金(檔案編號:0103/2021/A和0133/2022/A3)、深港澳科技計劃C類項目(檔案編號:SGDX20201103093600004)、何鴻燊博士醫療拓展基金會(檔案編號:SHMDF-OIRFS/2022/002)和澳門大學教育部精準腫瘤學前沿科學中心(SP2023-00001-FSCPO)資助。研究文章的完整版本可瀏覽https://pubs.acs.org/doi/full/10.1021/acsnano.3c02428。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/presss-release/detail/56537/
UM develops novel nanomedicine for augmenting cancer immunotherapy
A research team led by Dai Yunlu, associate professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), has made significant progress in cancer immunotherapy. The study fabricates a new metal-polyphenol nanomedicine to regulate cancer cell metabolism using ultrasound-mediated oxidative stress and aerobic glycolysis suppression. The ‘sono-metabolic’ treatment helps reprogramme the tumour microenvironment, thereby improving T-cell antitumour efficacy. The research results have been published in the international journal ACS Nano.
Cellular metabolism is crucial for maintaining the viability and function of cancer cells and immune cells. A hallmark property of malignancy is that, even under well-oxygenated conditions, cancer cells break down glucose to produce lactic acid by glycolysis, a phenomenon known as ‘aerobic glycolysis’. The study found that cancer cells with high metabolic activity outcompete immune cells for limited nutrients available in the surrounding environment. High glucose uptake and lactate production by cancer cells not only deprive CD8+ T cells of their energy source and impair their effector functions, but also support the immunosuppressive activity of regulatory T (Treg) cells.
In light of this, the research team developed a novel metal-phenolic nanomedicine, HPP-Ca@GSK, to perform efficient cancer cellular mitochondrial dysfunction and tumour microenvironment reprogramming via sono-metabolic treatment. Specifically, the team introduced the GSK2837808A (GSK, LDHA inhibitor) to suppress glycolytic metabolism in cancer cells and recreates a high-glucose, low-lactate tumour microenvironment, which is conducive to CD8+ tumour-infiltrating T lymphocyte effector function and Treg cell destabilisation. In addition, ultrasound-mediated oxidative stress induces intracellular calcium overload, which triggers the mitochondrial disorder for highly efficient damage-associated molecular patterns exposure. This assists dendritic cell maturation and CD8+ T cell activation, enabling more tumour infiltration of effective CD8+ T cells. Finally, the assistance of aCTLA-4 antibodies further induces the phenotypic instability of Treg cells and amplifies the sono-metabolic therapeutic influence.
Prof Dai and Li Bei, research assistant professor in the FHS, are the corresponding authors of the study. PhD student Yan Jie, research assistant Li Wenxi, and PhD student Tian Hao in the FHS are the co-first authors. PhD student Yu Xinying, PhD graduates Wang Guohao and Sang Wei made important contributions to the study. The Proteomics, Metabolomics and Drug Development Core, Animal Research Core, and Biological Imaging and Stem Cell Core of the FHS also provided support for the research. The research project was supported by the National Natural Science Foundation of China (File no: 32222090, 32171318, and 32101069), UM (File no: MYRG2022-00011-FHS), the Science and Technology Development Fund of the Macao SAR (File no: 0103/2021/A and 0133/2022/A3), the Shenzhen-Hong Kong-Macao Technology Research Programme (Type C) (File no: SGDX20201103093600004), Dr. Stanley Ho Medical Development Foundation (File no: SHMDF-OIRFS/2022/002) and the Ministry of Education Frontiers Science Center for Precision Oncology, University of Macau (File no: SP2023-00001-FSCPO). The full version of the research article is available at https://pubs.acs.org/doi/full/10.1021/acsnano.3c02428.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/press-release/detail/56537/