News Express: UM achieves progress in addressing heterogeneity of autism
新聞快訊:澳大在解決自閉症譜系障礙異質性取得進展
大腦內在網絡的功能連接強度的組間差異
Group differences in the mean functional connectivity (FC) of selected intrinsic networks
澳大在解決自閉症譜系障礙異質性取得進展
澳門大學認知與腦科學研究中心(CCBS)助理教授李日輝的研究團隊在解決自閉症譜系障礙(孤獨症,ASD)的異質性方面取得系列進展。團隊利用功能性磁共振成像(fMRI)和腦電圖(EEG)技術揭示了基於症狀和基因的孤獨症亞型的非典型腦連接組,為解決孤獨症的高異質性提供了新的思路,可用於對孤獨症的精準健康管理和治療干預。
孤獨症是最普遍的神經發育障礙疾病之一,影響約1%至2%的人口。被診斷為孤獨症的個體通常表現出嚴重的社交行為障礙和重複刻板行為,理解孤獨症潛在的神經機制一直是研究人員長期追求的目標。然而,在孤獨症人群中病因和行為表現的顯著異質性使得尋找該疾病的一致神經特徵變得相當困難。目前,領域內普遍認為孤獨症包含多種亞型,具有不同的病因和發展軌跡,研究這些亞型的獨特神經特徵有利於臨床上預測孤獨症症狀的發展,並推進孤獨症精準干預策略的研發。
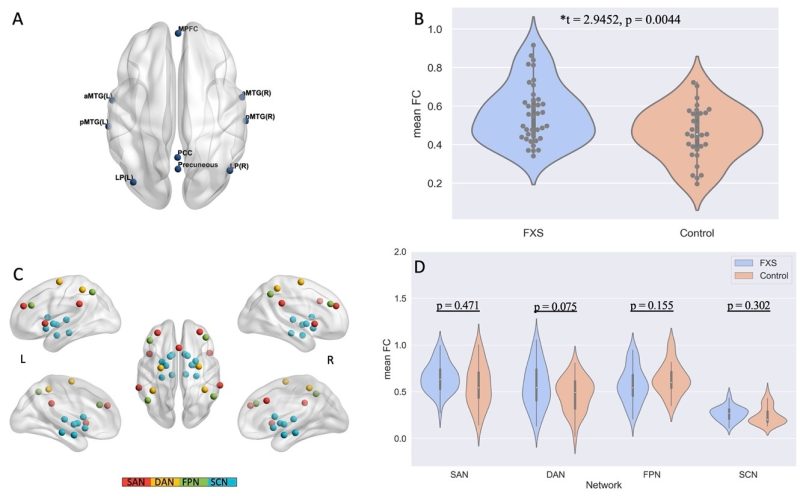
研究團隊的其中一項研究深入探討脆性X綜合症(FXS)患者的異常腦功能連接圖譜。FXS是與孤獨症高度相關的遺傳疾病,是孤獨症最常見的遺傳風險因素(約40%至50%的患者會被診斷為孤獨症)。研究團隊收集了38名被診斷為FXS的女性的靜息態功能性磁共振(fMRI)數據,並比較她們與32名在智商、社交功能和年齡上匹配的女性(對照組)。結果顯示,與對照組相比,患有FXS的女性顯示出大腦默認網絡的功能連接顯著增加(圖1),右顳中回的節點強度降低,左側尾狀核的節點強度增強,默認網絡的全局效率增加。這些異常的腦網絡特徵直接映射到FXS女性常見的認知行為症狀(圖2)。 探索性的縱向分析表明,較早時間點的腦網絡模式可以預測FXS患者多項認知行為症狀的縱向發展。這些發現為了解FXS對大腦功能連接的影響提供了重要的證據,並為FXS女性行為表型的發展提供了潛在的評價方法。該研究表明孤獨症不同的亞型和致病因素存在特異性的腦網絡連接圖譜,並已發表於著名精神病學期刊《Biological Psychiatry》。
李日輝是該研究的第一兼通訊作者,斯坦福大學教授Allan Reiss為資深作者。研究得到美國國家精神衛生研究所(檔案編號:R01MH050047和T32MH019908)、斯坦福大學Maternal & Child Health Research Institute(檔案編號:1220552-152-DHPEU)、澳門大學研究啟動基金(檔案編號:SRG2023-00015-ICI)和Canel家庭基金資助。全文可瀏覽:https://www.biologicalpsychiatryjournal.com/article/S0006-3223(23)01168-X/fulltext
此外,團隊在另一項研究中提出了一種基於臨床症狀的孤獨症亞型識別方法,探索了孤獨症亞型的靜息態腦功能網絡連接圖譜。團隊收集了72名被診斷為孤獨症的兒童和63名性別和年齡匹配的典型發育兒童的靜息態腦電(EEG)數據,以及多項孤獨症行為症狀量表。該研究利用聚類算法,基於孤獨症患者多領域的自閉症症狀臨床量表分數(SRS-2和Vineland-3)鑒定出兩個孤獨症亞型:症狀更嚴重的sASD和癥狀較輕的mASD(圖3)。研究對他們EEG數據的後續分析揭示了上述兩個亞組的靜息狀功能連接的不同特點。具體來說,mASD組顯示出Beta波段中功能連接的增加,而sASD組在Alpha波段中顯示出功能連接的減少。在Alpha和Beta波段中都發現了全腦和局部腦區拓撲結構的顯著組間差異(圖4)。這些結果提示了症狀嚴重程度的差異可能是前人關於孤獨症兒童功能連接的研究存在不一致性的原因,為解決孤獨症研究中的異質性提供了新的視角,相關成果已發表於著名神經科學期刊《Cerebral Cortex》。
該研究由李日輝和解放軍總醫院教授楊光共同領導,澳大博士生李宇航和解放軍總醫院研究生朱剛為共同第一作者。研究得到澳門大學研究啟動基金(檔案編號:SRG2023-00015-ICI)、北京自然科學基金一般專案(檔案編號:7222187)和軍隊計劃生育特殊科研專案(檔案編號:22JSZ20)資助。全文可瀏覽: https://doi.org/10.1093/cercor/bhad413
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/campus-news/detail/57392/
UM achieves progress in addressing heterogeneity of autism
Li Rihui, assistant professor at the Centre for Cognitive and Brain Sciences of the University of Macau (UM), and his research team have achieved significant breakthroughs in addressing the heterogeneity of autism spectrum disorder (ASD). By using functional magnetic resonance imaging (fMRI) and electroencephalogram (EEG) technologies, the team has revealed the atypical brain connectome of both symptom-based and genetic-based ASD subtypes. This provides a new perspective to address the heterogeneity in ASD research and can be applied to precise healthcare management and therapeutic interventions for individuals with ASD.
As one of the most prevalent neurodevelopmental disorders, ASD affects approximately 1 to 2 per cent of the population. Individuals diagnosed with ASD typically exhibit challenges in social communication and restricted repetitive behaviours. Understanding the intricate neural mechanisms underlying ASD has been a longstanding goal for researchers. However, the substantial heterogeneity in both causes and manifestations within the ASD population has posed a considerable challenge in finding a consistent neural signature for the condition. A consensus has emerged that ASD comprises various subgroups with diverse etiologies and developmental trajectories. Investigating the distinctive characteristics of these subgroups therefore offers promise in predicting symptom development and advancing precision intervention strategies for ASD.
One of the studies by the research team delved into the atypical functional connectivity patterns of patients with Fragile X syndrome (FXS). FXS is a genetic disorder frequently associated with ASD, serving as one of the most common heritable risk factors of ASD (about 40 to 50 per cent of patients with FXS will be diagnosed with ASD). The team collected resting-state fMRI data from 38 girls diagnosed with FXS, and compared them to 32 girls (control group) matched in IQ, social function, and age. Their results showed that compared with the control group, girls with FXS showed significantly greater resting-state functional connectivity of the default mode network, lower nodal strength at the right middle temporal gyrus, stronger nodal strength at the left caudate, and higher global efficiency of the default mode network. These aberrant brain network characteristics map directly onto the cognitive behavioural symptoms commonly observed in girls with FXS. An exploratory analysis suggested that brain network patterns at a prior time point were predictive of the longitudinal development of participants’ multidomain cognitive behavioural symptoms. These findings contribute valuable insights into the impact of FXS on the brain’s connectome and offer a potential tool for assessing the developmental trajectory of behavioural phenotypes in girls with FXS. Moreover, the study highlights the specific brain connectome patterns linked to the different subtypes and risk factors of ASD. The study has been published in Biological Psychiatry, a leading journal in psychiatry.
Prof Li is the first and corresponding author of the research, with Allan Reiss, professor at Stanford University, as the senior author. The research is supported by the National Institute of Mental Health in the US (File no: R01MH050047 and T32MH019908), the Maternal & Child Health Research Institute at Stanford University (File no: 1220552-152-DHPEU), the Start-up Research Grant of UM (File no: SRG2023-00015-ICI), and the Canel Family Fund. The full version of the article can be viewed at https://www.biologicalpsychiatryjournal.com/article/S0006-3223(23)01168-X/fulltext.
In another study, the team employed a symptom-based subgroup identification method to explore the resting-state functional connectivity of ASD subgroups. They collected resting state EEG data and evaluated autism behavioural symptom scores from 72 children diagnosed with ASD, alongside 63 typically developing children matched in gender and age. Using this approach, which was based on clustering and participants’ multidomain autism symptom clinical scale scores (SRS-2—Social Responsiveness Scales-Second Edition, and Vineland-3—Vineland Adaptive Behavior Scales-Third Edition), two ASD subgroups were identified: one with more severe symptoms (sASD) and another with milder symptoms (mASD). Subsequent analysis of their EEG data uncovered divergent abnormalities in the resting-state functional connectivity of these two subgroups. Specifically, mASD group displayed increased functional connectivity in the beta band, while sASD group exhibited decreased connectivity in the alpha band. Significant between-group differences in global and regional topological abnormalities were identified in both alpha and beta bands. These results imply that variations in symptom severity might account for previous inconsistencies in research regarding the functional connectome of ASD children. The study has introduced a fresh perspective for addressing heterogeneity in ASD research and has been published in Cerebral Cortex, a leading journal in neuroscience.
This research is co-led by Prof Li and the team of Prof Yang Guang from the Chinese People’s Liberation Army (PLA) General Hospital. Li Yuhang, a doctoral student at UM, and Zhu Gang, a postgraduate student at the Chinese PLA General Hospital, are the co-first authors of the research. The research is supported by the Start-up Research Grant of UM (File no: SRG2023-00015-ICI), the General Project of Beijing Natural Science Foundation (File no: 7222187), and the Special Scientific Research Project of Military Family Planning (File no: 22JSZ20). The full version of the article can be viewed at https://doi.org/10.1093/cercor/bhad413.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/campus-news/detail/57392/