News Express: UM’s stem cell research discovers new mechanism for anti-tumour immunity
新聞快訊:澳大幹細胞研究揭示抗腫瘤免疫新機制
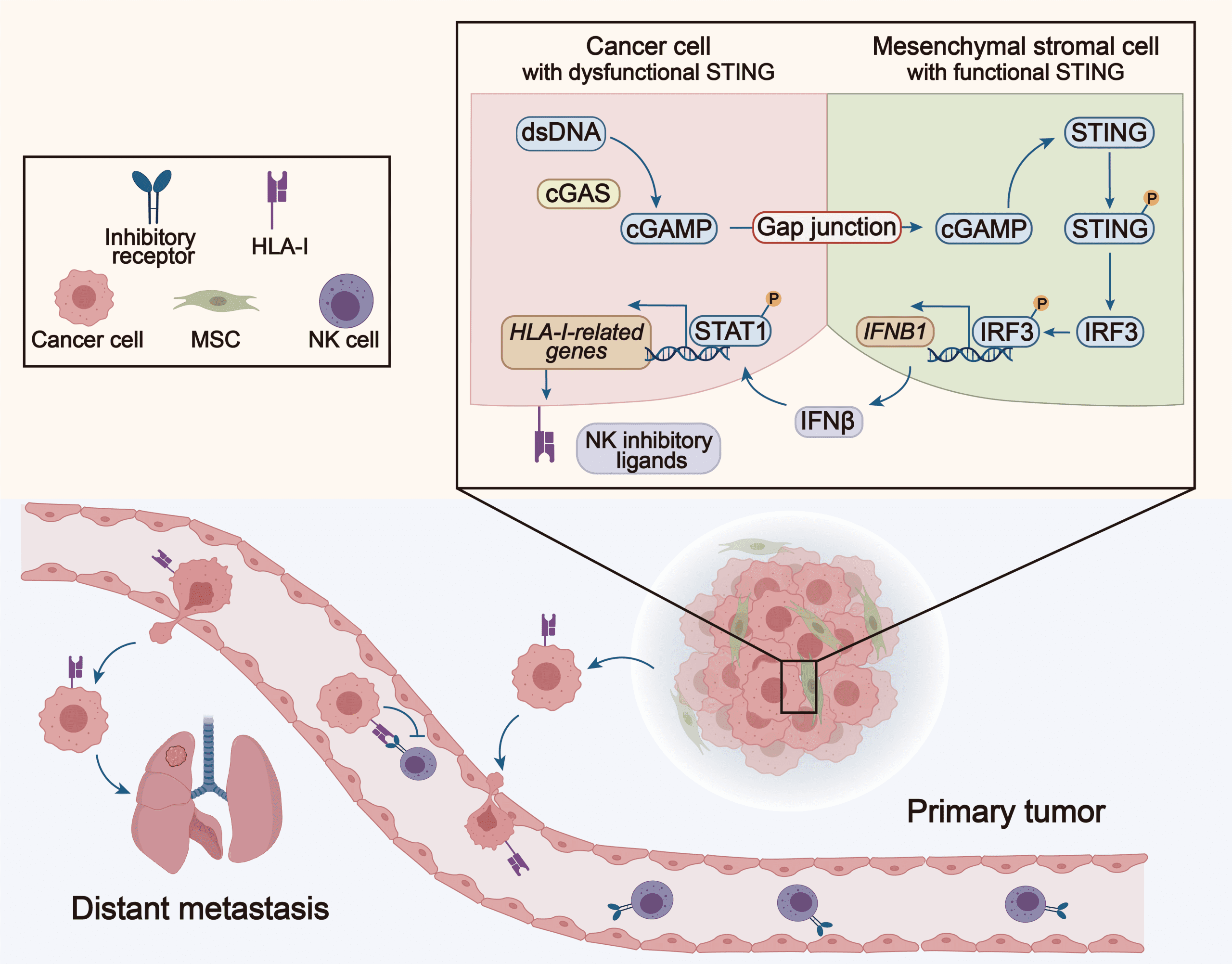
間充質基質細胞幫助腫瘤細胞逃逸自然殺傷細胞的示意圖
A schematic illustration of how mesenchymal stromal cells help cancer cells escape natural killer cells
澳大幹細胞研究揭示抗腫瘤免疫新機制
澳門大學健康科學學院特聘教授徐仁和領導的研究團隊,在腫瘤逃避免疫系統識別和攻擊的機制探索上取得重要進展。研究團隊利用幹細胞模擬腫瘤微環境(TME)中的間充質基質細胞(MSC),發現MSC能通過與腫瘤細胞相互作用,產生一系列細胞間信號,從而提高腫瘤細胞對自然殺傷(NK)細胞的抵抗力,促進腫瘤轉移。該發現已在臨床樣本中取得驗證,揭示了一種新的腫瘤免疫逃逸機制,為抗腫瘤免疫治療提供了新的靶點。相關成果已在國際知名期刊《先進科學》(Advanced Science)上發表。
腫瘤生長過程中會產生免疫抑制環境,腫瘤細胞可通過募集抑制性免疫細胞及分子,形成一個免疫抑制性的腫瘤微環境(TME),從而逃避宿主的免疫系統。研究顯示,作為TME的重要組成成分,MSC不僅能促進腫瘤細胞的生長、侵襲和轉移,還能抑制NK細胞和T細胞等免疫細胞的功能,因此在腫瘤進展中扮演著關鍵角色。然而,關於MSC是否能夠直接調控腫瘤細胞對免疫細胞抵抗力的研究還相對不足。
研究團隊發現,在腫瘤細胞與MSC共培養後,腫瘤細胞對NK細胞的抵抗力顯著增強。進一步的轉錄組測序分析顯示,共培養的腫瘤細胞表達了更多的NK抑制性配體,這有助於腫瘤細胞抵抗NK細胞攻勢。值得注意的是,這種現象依賴於細胞之間的直接接觸。當MSC和腫瘤細胞非接觸培養或阻斷它們之間的間隙連接時,NK細胞的抵抗力隨之消失。進一步研究發現,腫瘤細胞的細胞質中含有大量的雙鏈DNA(dsDNA),這些dsDNA能被cGAS識別,並合成第二信使cGAMP。cGAMP通過間隙連接傳遞到周圍的MSC中,啟動STING信號通路,誘導IFNβ產生。IFNβ通過旁分泌作用於腫瘤細胞,提高NK抑制性配體的表達,從而增強腫瘤細胞的NK抵抗性。在乳腺癌、非小細胞肺癌和胰腺癌患者中,IFN受體及NK抑制性配體的表達水平和患者的總生存期呈顯著負相關。因此,該研究表明腫瘤細胞和MSC的相互作用及其觸發的信號通路,可能成為未來腫瘤治療的新靶點。
該研究的通訊作者為徐仁和,第一作者為澳大健康科學學院博士生易曄。該院院長鄧初夏、教授羅茜和副教授劉子銘,以及博士後賈皓、楊紅梅和曾奇兵,博士生覃貴慧、葉森、鄭德景和張志明也在該研究中做出了重要的貢獻。健康科學學院的核心實驗中心,特別是動物中心和生物成像及幹細胞中心也為研究提供了重要支持。此項研究得到了科技部國家重點研發項目(檔案編號:2022YFA1105000)、國家自然科學基金(檔案編號:32270842)、澳門科學技術發展基金(檔案編號:0002-2021-AKP和0071-2022-A2)和澳大(檔案編號:CPG2024-00037-FHS、MYRG2020-00140-FHS和MYRG2022-00044-FHS)的資助。研究文章的完整版本可瀏覽https://onlinelibrary.wiley.com/doi/10.1002/advs.202400888。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/presss-release/detail/58440/
UM’s stem cell research discovers new mechanism for anti-tumour immunity
A research team led by Xu Ren-He, Distinguished Professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), has made important progress in understanding the mechanisms of tumour immune evasion. They used stem cells to simulate mesenchymal stromal cells (MSCs) in the tumour microenvironment (TME), and found that MSCs can produce a cascade of intercellular signals by interacting with tumour cells, thereby enhancing the resistance of tumour cells against natural killer (NK) cells and promoting tumour metastasis. These findings, validated in clinical samples, reveal a novel mechanism of tumour immune evasion and provide a new therapeutic target for anti-tumour immunotherapy. The research findings have been published in the internationally renowned journal Advanced Science.
During tumour progression, cancer cells can evade the host immune system by recruiting inhibitory immune cells and secreting cytokines to form an immunosuppressive TME. Studies have demonstrated that MSCs, as an essential component of the TME, not only promote tumour cell proliferation, invasion, and metastasis, but also inhibit the functions of immune cells such as NK cells and T cells. Therefore, MSCs are considered to play a crucial role in tumour progression. However, there has been little research into the ability of MSCs to directly regulate tumour cell resistance to immune cells.
The research team observed that after coculturing with MSCs, the cancer cells exhibited an increased resistance to NK cell cytotoxicity. Further transcriptome analysis revealed that cocultured tumour cells expressed higher levels of NK inhibitory ligands, which helped the tumour cells escape NK cell-mediated cytotoxicity. It is noteworthy that this phenomenon is dependent on direct coculture between the cells. When MSCs and tumour cells were co-cultured indirectly or when gap junctions between the two cell types were blocked, the NK resistance disappeared. Further studies showed that abundant double-stranded DNA (dsDNA) is accumulated in the cytoplasm of tumour cells, which can be recognised by cGAS. cGAS then synthesises the second messenger cGAMP, which is transferred through gap junctions to surrounding MSCs, thereby activating the STING signalling pathway and inducing IFNβ production. IFNβ binds to tumour cells and upregulates their expression of NK inhibitory ligands, thereby enhancing tumour cell resistance to NK cells. In patients with breast cancer, non-small cell lung cancer and pancreatic cancer, expression levels of IFN receptors and the NK inhibitory ligands are significantly and negatively correlated with overall survival. Therefore, the study suggests that the interaction between tumour cells and MSCs, and the signalling pathway triggered by this interaction, may become a new target for tumour therapy.
The corresponding author of the paper is Prof Xu, and the first author is Yi Ye, a PhD student in FHS. FHS Dean Chuxia Deng, Professor Luo Qian, Associate Professor Liu Tzu-Ming, postdoctoral fellows Jia Hao, Yang Hongmei and Zeng Qibing, and PhD students Qin Guihui, Ye Sen, Zheng Dejin and Zhang Zhiming also made significant contributions to the study. All the core facilities of FHS, especially the Animal Research Core and the Biological Imaging and Stem Cell Core, provided substantial support for the research. The research project was supported by the National Key R&D Program of the Ministry of Science and Technology (File no: 2022YFA1105000), the National Natural Science Foundation of China (File no: 32270842), the Science and Technology Development Fund of the Macao SAR (File no: 0002-2021-AKP and 0071-2022-A2), and UM (File no: CPG2024-00037-FHS, MYRG2020-00140-FHS, and MYRG2022-00044-FHS). The full version of the research article is available at https://onlinelibrary.wiley.com/doi/10.1002/advs.202400888.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/presss-release/detail/58440/