News Express: UM develops novel oral formulation for ulcerative colitis
新聞快訊:澳大研發創新結腸炎口服藥
澳大研發創新口服藥物精準治療潰瘍性結腸炎
UM develops a novel oral formulation for the targeted therapy of ulcerative colitis
澳大研發創新結腸炎口服藥
澳門大學中華醫藥研究院王瑞兵教授的研究團隊開發了一種創新的口服藥物,能夠精準靶向治療潰瘍性結腸炎。該研究成果已於國際頂級學術期刊《科學進展》(Science Advances)刊登。
潰瘍性結腸炎是一種常見的炎症性腸病,影響著胃腸道。雖然口服抗炎藥物是臨床首選,但是由於藥物積聚不足、黏膜屏障滲透有限、以及過量的活性氧和炎症細胞因子難以清除等複雜挑戰的阻礙,臨床效果並不理想。因此,開發一種能有效滲透黏膜屏障、靶向積聚於炎性組織並高效清除炎症因子和活性氧的口服制劑至關重要。
研究團隊此前已開發了巨噬細胞仿生納米藥物和巨噬細胞海綿高效靶向治療炎性疾病,如動脈粥樣硬化、急性肺炎等,並先後在《自然通訊》(Nature Communications)上發表。但此兩項成果均為注射劑型,無法口服治療腸炎,且缺乏主動穿透黏膜屏障的功能。
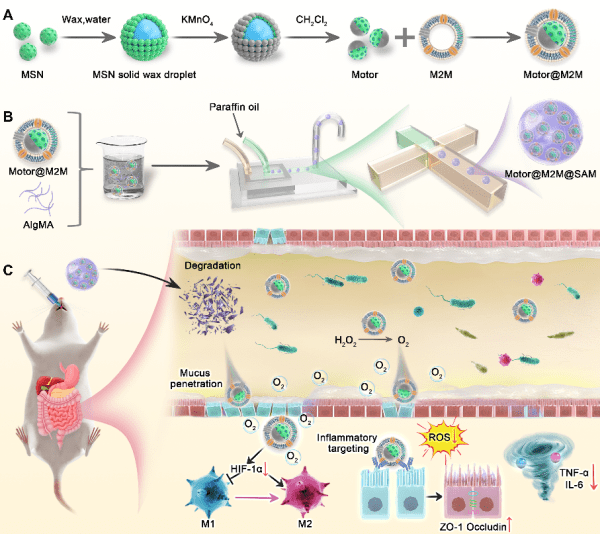
基於此,研究團隊最近開發了一種不含小分子藥物的口服製劑。該製劑使用M2巨噬細胞膜塗層的非對稱納米馬達(M2巨噬細胞仿生納米機器人),並將其嵌入海藻酸鈉水凝膠微球中。海藻酸鈉微球確保了納米馬達在惡劣的胃液環境中的穩定性,並將其釋放到腸道中。M2巨噬細胞膜提高了納米馬達進入炎性結腸的遞送效率,同時作為納米海綿高效中和炎性因子。此外,納米馬達通過催化過氧化氫產生的氧氣的推進力,穿過結腸黏膜屏障進入炎性組織。這一過程清除活性氧,緩解組織乏氧,極化巨噬細胞,並減輕結腸上皮細胞的凋亡。
相關研究成果以“仿M2巨噬細胞‘雙面神’納米馬達的口服微球製劑靶向治療潰瘍性結腸炎”為題發表於綜合性知名期刊《科學進展》(Science Advances)。該論文通訊作者為澳大中華醫藥研究院教授王瑞兵,共同通訊作者為澳大中華醫藥研究院研究助理教授高成,澳大博士生羅銳鋒、碩士生劉晉瑋,以及澳大博士畢業生、現成都中醫藥大學副教授成謙為文章共同第一作者。此項研究由澳門特別行政區科學技術發展基金(檔案編號:0047/2023/AMJ、0086/2022/A2、005/2023/SKL、0001/2023/RIA1、0070/2023/RIA2和0006/2022/ITP)及澳門大學(檔案編號:MYRG2022-00081-ICMS)資助。研究文章全文可瀏覽https://www.science.org/doi/10.1126/sciadv.ado6798。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/presss-release/detail/58597/
UM develops novel oral formulation for ulcerative colitis
A research team led by Wang Ruibing, professor at the Institute of Chinese Medical Sciences (ICMS) at the University of Macau (UM), has developed a novel oral formulation for the targeted therapy of ulcerative colitis. The research results have been published in the prestigious international journal Science Advances.
Ulcerative colitis, a type of inflammatory bowel disease, is a common inflammatory disease of the gastrointestinal tract. Although oral anti-inflammatory medications are the preferred method for patients, clinical outcomes are often unsatisfactory due to challenges including poor drug accumulation, limited penetration of mucus barrier, and insufficient elimination of reactive oxygen species (ROS) and inflammatory cytokines. Therefore, it is critical to develop an oral formulation that can effectively penetrate the mucus barrier, accumulate in inflamed tissue, and eliminate inflammatory factors and ROS.
The research team had previously developed macrophage-based nanodrugs and macrophage sponges for the targeted therapy of inflammatory diseases such as atherosclerosis and acute pneumonia, and had published two research articles in Nature Communications. However, both of them are injectable formulations that are not suitable for oral administration to treat intestinal inflammation and are unable to actively penetrate the mucus barrier.
Building on the previous research, the research team then developed an oral medication that does not contain small-molecule drugs. This formulation uses asymmetric M2 macrophage membrane-coated Janus nanomotors (M2 macrophage-biomimetic nanorobots) embedded in sodium alginate hydrogel microspheres. The sodium alginate microspheres ensure the stability of the nanomotors in the harsh gastric environment and release them into the intestinal tract. The M2 macrophage membrane enhances the delivery efficiency of the nanomotors to the inflammatory colon and acts as a nanosponge to effectively neutralise inflammatory factors. Additionally, the propelling force of oxygen generated by the catalytic consumption of hydrogen peroxide facilitates the penetration of nanomotors through the colonic mucus barrier into inflammatory tissues. This process eliminates ROS, alleviates tissue hypoxia, polarises macrophages, and reduces colonic epithelial cell apoptosis.
The research is published in the prestigious journal Science Advances under the title ‘Oral microsphere formulation of M2 macrophage-mimetic Janus nanomotor for targeted therapy of ulcerative colitis’. Prof Wang is the corresponding author, and Gao Cheng, research assistant professor at ICMS, is the co-corresponding author. UM PhD candidate Luo Ruifeng, MPhil student Liu Jinwei, and Cheng Qian, PhD graduate of UM and associate professor at Chengdu University of Traditional Chinese Medicine, are the co-first authors. The research project was funded by the Science and Technology Development Fund of the Macao SAR (File No: 0047/2023/AMJ, 0086/2022/A2, 005/2023/SKL, 0001/2023/RIA1, 0070/2023/RIA2, and 0006/2022/ITP) and UM (File No: MYRG2022-00081-ICMS). The full version of the research article is available at https://www.science.org/doi/10.1126/sciadv.ado6798.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/presss-release/detail/58597/