News Express: UM research makes groundbreaking discovery in study of breast cancer metastasis routes
新聞快訊:澳大研究乳腺癌細胞轉移路徑有重大發現
單細胞條碼追蹤技術揭示乳腺癌細胞轉移路徑
Single-cell barcoding technology visualises breast cancer metastasis routes
澳大研究乳腺癌細胞轉移路徑有重大發現
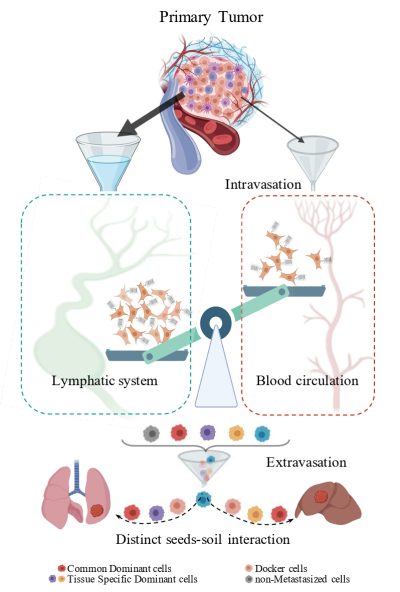
澳門大學健康科學學院講座教授鄧初夏和助理教授苗凱的研究團隊在癌症轉移研究領域取得重大進展。該研究首次運用單細胞條碼追蹤技術(CellTag)對乳腺癌細胞的轉移路徑進行定性和定量分析,結果揭示癌細胞主要通過淋巴系統而非血液循環實現遠端轉移,並發現腫瘤細胞與微環境的動態交互作用是決定細胞命運和驅動轉移的關鍵機制。研究系統展示了腫瘤轉移的途徑,為癌症治療策略的開發提供了全新方向,相關成果已發表於國際知名期刊《分子癌症》(Molecular Cancer)。
癌症轉移是導致患者死亡的主要原因,但腫瘤細胞如何選擇轉移路徑仍不明確。研究團隊利用單細胞條碼標記技術,追蹤小鼠模型中乳腺癌細胞的遷移路徑。結果顯示,僅少於3%的腫瘤細胞通過血液循環擴散,而超過80%的轉移細胞經由淋巴系統擴散至肺、肝等器官。進一步分析發現,淋巴轉移的腫瘤細胞具有獨特的基因表達模式,並與微環境中的基質細胞(如纖維母細胞、內皮細胞)通過細胞因子密切互動,形成“種子—土壤”適應性機制。
該研究從證據學角度揭示了癌症轉移的路徑偏好,並首次證實腫瘤細胞在轉移前已被“預編程”,具備決定未來轉移能力及目標器官的信息;同時,癌細胞能根據目標器官的微環境動態調整自身基因表達。該發現為開發針對淋巴轉移的特異性療法奠定了理論基礎,例如通過抑制關鍵細胞因子信號或阻斷腫瘤—基質細胞交互作用來遏制轉移。
此外,研究團隊結合單細胞RNA測序技術,發現同一來源的腫瘤細胞在不同器官中表現出顯著的基因差異,進一步證實微環境對腫瘤細胞行為的塑造作用。例如,轉移至肺部的細胞上調與氧化應激相關的基因,而肝轉移細胞則激活代謝調控通路。
該研究由澳大健康科學學院癌症中心主導,鄧初夏和苗凱為通訊作者。該項目獲澳門特別行政區科學技術發展基金(檔案編號:0073/2021/A2、111/2017/A、0007/2021/AKP、0009/2022/AKP、0004/2021/AKP和0065/2022/A2)及澳門大學(檔案編號:MYRG-GRG2023-00150-FHS-UMDF和MYRG-GRG2024-00146-FHS)資助。全文可瀏覽:https://doi.org/10.1186/s12943-025-02279-w。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/press-release/detail/61294/
UM research makes groundbreaking discovery in study of breast cancer metastasis routes
A research team led by Chair Professor Chuxia Deng and Assistant Professor Miao Kai in the Faculty of Health Sciences (FHS) at the University of Macau (UM) has made a groundbreaking discovery in the study of cancer metastasis. The team, for the first time, conducted a qualitative and quantitative analysis of breast cancer metastasis routes by applying a barcoding system based on a single-cell lineage tracing approach (CellTag). The results revealed that cancer cells primarily disseminate via the lymphatic system rather than the bloodstream. The research also found that crosstalk between tumour cells and the tumour microenvironment (TME) determines the metastatic potential of cancer cells. The study systematically demonstrates tumour metastatic routes and opens up new avenues for the development of cancer treatment strategies. The research has been published in the prestigious journal Molecular Cancer.
Cancer metastasis is the primary cause of cancer-related death, yet the mechanisms that govern metastatic route selection remain elusive. Using CellTag technology, the research team tracked the dissemination of breast cancer cells in mouse models. They found that less than 3% of cancer cells spread through the bloodstream, while over 80% metastasised via the lymphatic system to distant organs, such as the lungs and liver. Further analysis showed that the lymph-disseminated cells have distinct gene expression patterns and communicate closely with stromal cells (such as fibroblasts and endothelial cells) in the microenvironment, forming a ‘seed-soil’ adaptive mechanism.
The study provides empirical evidence for route preference in cancer metastasis and demonstrates that tumour cells are ‘prewired’ with intrinsic information that determines their metastatic potential and organotropism. Additionally, these tumour cells dynamically adapt their gene expression based on the microenvironment of the target organs. These findings lays the foundation for the development of therapies targeting lymphatic metastasis, such as the inhibition of cytokine signalling or the disruption of tumour-stroma crosstalk to suppress metastasis.
Moreover, by using single-cell RNA sequencing technology, the research team found that genetically identical tumour cells exhibit organ-specific gene expression profiles. This further demonstrates the pivotal role of the microenvironment in shaping tumour cell behaviour. For example, lung metastatic cells upregulate oxidative stress-related genes, while liver metastatic cells activate metabolic regulatory pathways.
The study was led by the Cancer Centre of UM FHS, with Prof Deng and Prof Miao serving as the corresponding authors. The research was funded by the Science and Technology Development Fund of the Macao SAR (File No.: 0073/2021/A2, 111/2017/A, 0007/2021/AKP, 0009/2022/AKP, 0004/2021/AKP, and 0065/2022/A2), and UM (File No.: MYRG-GRG2023-00150-FHS-UMDF and MYRG-GRG2024-00146-FHS). The full version of the research article is available at: https://doi.org/10.1186/s12943-025-02279-w.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/press-release/detail/61294/