News Express: UM research team discovers new method to inhibit triple-negative breast cancer
新聞快訊:澳大研究團隊發現有效抑制三陰性乳腺癌新方法
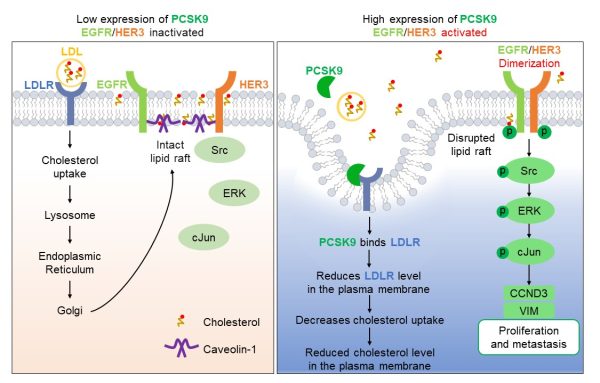
高表達PCSK9能夠激活EGFR和HER3介導的信號通路,從而促進TNBC細胞的惡性增殖與轉移(右圖)
High expression of PCSK9 activates EGFR and HER3-mediated signalling pathway to promote malignant proliferation and metastasis in TNBC cells (right)
澳大研究團隊發現有效抑制三陰性乳腺癌新方法
澳門大學健康科學學院教授羅茜的研究團隊揭示了三陰性乳腺癌(TNBC)惡性進展的突破性機制,為靶向治療開闢了新方向。研究首次發現,前蛋白轉化酶枯草溶菌素9(PCSK9)通過耗竭細胞膜膽固醇水平,激活致癌信號通路驅動TNBC進展。該突破性成果表明,抑制PCSK9或其下游效應可顯著抑制TNBC腫瘤生長與轉移。相關研究已發表於國際頂級期刊《先進科學》。
TNBC因缺乏雌激素受體、孕激素受體及HER2表達,是乳腺癌中侵襲性最強、治療選擇最有限的亞型,且轉移率極高。儘管膽固醇代謝與癌症進展相關,但作為膽固醇穩態關鍵調控因子的PCSK9,其在TNBC中的作用機制此前尚未明確。
團隊通過向小鼠注射親本MDA-MB-231細胞,從其肺轉移瘤中分離出高轉移性TNBC細胞(4-11)。RNA測序顯示,轉移性細胞中PCSK9表達顯著上調。實驗證實,基因沉默PCSK9可使腫瘤生長和轉移大幅減少,而過表達PCSK9則顯著增強惡性表型。
機制研究表明,PCSK9通過與低密度脂蛋白受體(LDLR)結合,減少膽固醇攝取,導致細胞膜膽固醇耗竭。這種膽固醇缺失會破壞脂筏結構,促使EGFR和HER3受體激活,進而驅動Src/ERK/c-Jun信號級聯反應,上調細胞周期蛋白D3和波形蛋白表達,最終促進腫瘤增殖與轉移。研究團隊通過使用靶向EGFR/HER3及相關下游激酶的抑制劑與激動劑,驗證了該信號傳導軸,證實了PCSK9驅動的惡性腫瘤行為的核心作用。值得注意的是,人工耗竭膜膽固醇或LDLR可產生類似促轉移效應,證實脂筏破壞是致癌通路激活的關鍵觸發因素。臨床數據分析進一步揭示,PCSK9高表達的TNBC患者生存預後顯著更差。該研究通過整合細胞生物化學與臨床證據,為TNBC精準治療提供了全新策略。
羅茜為該研究的通訊作者,澳大健康科學學院博士畢業生李天紅、吳仁飛分別為第一及第二作者。該研究獲澳門特別行政區科學技術發展基金(檔案編號:0147/2020/A3和0004/2021/AKP)及國家教育部澳門大學精準腫瘤學前沿科學中心(檔案編號:SP2021-00001-FSCPO和SP2023-00001-FSCPO)資助。全文可瀏覽:https://doi.org/10.1002/advs.202408514。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/campus-news/detail/61355/
UM research team discovers new method to inhibit triple-negative breast cancer
A research team led by Kathy Luo Qian, professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), has unveiled a groundbreaking mechanism driving the malignancy of triple-negative breast cancer (TNBC), opening new avenues for targeted therapies. The study revealed that proprotein convertase subtilisin/kexin type 9 (PCSK9) promotes TNBC progression by depleting cholesterol levels in the plasma membrane, thereby activating oncogenic signalling pathways. These groundbreaking findings suggest that inhibiting PCSK9 or its downstream effectors could significantly suppress TNBC tumour growth and metastasis. The research has been published in the internationally renowned journal Advanced Science.
TNBC, characterised by the absence of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2), is the most aggressive breast cancer subtype with limited treatment options and a high metastatic rate. Although cholesterol metabolism has been associated with cancer progression, the role of PCSK9, a key regulator of cholesterol homeostasis, in TNBC was unclear prior to this study.
The team isolated highly metastatic TNBC cells (4-11) from the lung tumours of mice that had been injected with parental MDA-MB-231 cells. Subsequent RNA sequencing analysis revealed a significant upregulation of PCSK9 in the metastatic cells. The experiment demonstrated that silencing PCSK9 drastically reduced tumour growth and metastasis, while overexpressing PCSK9 enhanced these processes.
Mechanistically, binding PCSK9 to the low-density lipoprotein receptor (LDLR) reduces cholesterol uptake, leading to cholesterol depletion in the plasma membrane. The depletion of cholesterol disrupts lipid rafts, enabling the activation of epidermal growth factor receptor (EGFR) and HER3. These receptors then drive the Src/ERK/c-Jun signalling cascade, which upregulates cyclin D3 and vimentin to promote cell proliferation and metastasis. The team further validated these findings using inhibitors and activators that target EGFR/HER3 and related downstream kinases. This conclusively demonstrated the pivotal role of PCSK9-driven malignancy. Notably, the artificial depletion of membrane cholesterol or LDLR produced similar pro-metastatic effects, confirming that disruption of lipid rafts is a key trigger for the activation of oncogenic pathways. Analysis of clinical data revealed that TNBC patients with high PCSK9 expression had significantly lower survival rates. By combining cellular biochemistry with clinical evidence, the study offers new strategies for precision treatment of TNBC.
Prof Luo is the corresponding author of the study. FHS PhD graduates Li Tianhong and Wu Renfei are the first and second authors, respectively. The research was funded by the Science and Technology Development Fund (FDCT) of the Macao SAR (File No.: 0147/2020/A3 and 0004/2021/AKP), and the Ministry of Education Frontiers Science Center for Precision Oncology, University of Macau (File No.: SP2021-00001-FSCPO and SP2023-00001-FSCPO). The full version of the research article is available at: https://doi.org/10.1002/advs.202408514.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/campus-news/detail/61355/