News Express: UM research team establishes biobank of living tumour tissues to support personalised treatment and research
新聞快訊:澳大團隊建立活腫瘤組織標本庫 助力個體化精準治療及研究
澳大健康科學學院研究團隊建立活腫瘤組織標本庫
UM FHS research team establishes a biobank of living tumour tissues
澳大團隊建立活腫瘤組織標本庫 助力個體化精準治療及研究
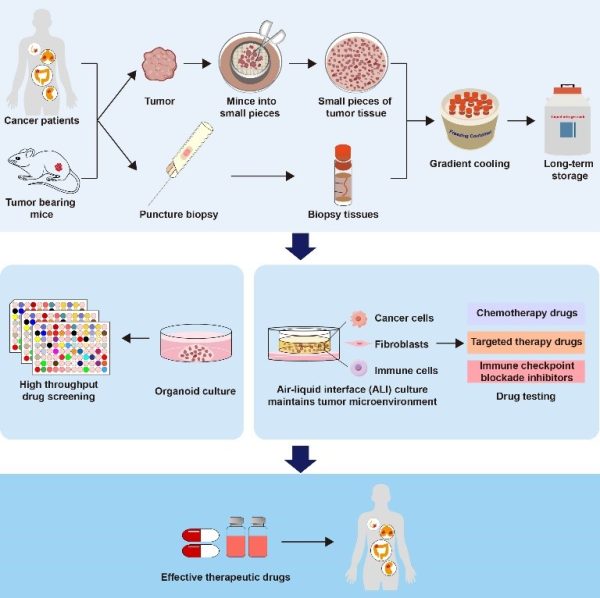
澳門大學健康科學學院講座教授鄧初夏帶領的研究團隊聯合鏡湖醫院團隊建立活腫瘤組織標本庫,通過標準化技術冷凍早期外科手術中獲得的腫瘤組織標本,在復發或轉移過程中腫瘤樣本無法獲得的情況下,冷凍組織可以被復甦用於培養,為腫瘤患者提供潛在的個性化治療或用於研究。相關研究成果已發表於國際學術期刊《生物活性材料》上。
生物樣本庫包括收集和儲存大量生物樣本,以及相關的個人資料,如健康記錄、家族史、生活方式資訊和遺傳資料,以促進健康和醫學研究工作。近年來,大規模生物樣本庫的建立呈明顯上升趨勢,包括腫瘤組織和其他樣本。這些細胞庫通常直接在冷凍條件下儲存標本,即超低溫冰箱或液氮。然而,相關儲存方法大多會殺死細胞,導致標本只能用於提取蛋白質、DNA或RNA,而不能用於培養活細胞,顯著限制了它們的整體價值和潛在應用。
面對這一挑戰,澳大研究團隊提出了“建立活腫瘤組織標本庫”的新設想,通過標準化技術,逐漸冷卻組織,並轉移至液氮中長期保存。該研究重新定義了組織生物樣本庫的概念,不僅能夠利用冷凍保存的組織培養來源於患者的腫瘤類器官(PDOs),還支援氣液介面(ALI)培養,並保留了原始腫瘤的免疫微環境。該成果解決了將死腫瘤庫轉化為活腫瘤庫的難題,有望應用於腫瘤個體化精準治療及研究。
研究團隊對42對冷凍的人類腫瘤標本進行了測試,將來自乳腺癌、結直腸癌、肺癌、腎癌等四種不同癌症類型的組織樣本用於建立患者來源的類器官模型。研究結果顯示,除兩例凍存標本類器官培養失敗外,其餘40例均成功,成功率為95.2%。值得注意的是,即使在冷凍近四年後,腫瘤樣本仍可用於類器官模型的建立。此外,研究明確證明了該技術在有效冷凍保存微小標本(包括活檢組織)中的適用性。
該活體組織冷凍保存技術具有簡單易操作等優點,可以維持不同的腫瘤標本進行長期冷凍,同時保留腫瘤組織內細胞的活力。來源於凍存腫瘤組織的類器官也可以持續凍存和傳代,能夠進行高通量藥物敏感性篩選和鑒定潛在有效的藥物來對抗耐藥腫瘤。另外,冷凍保存的腫瘤組織保留了原始的腫瘤微環境,也適用於氣液模型的培養,能夠評價包括免疫檢查點抑制劑在內的抗腫瘤藥物反應。該研究擴展了生物樣本庫概念,為建立活組織樣本庫奠定了基礎,將為癌症精準治療和轉化醫學研究提供巨大支援。
該研究通訊作者為鄧初夏,第一作者為澳大健康科學學院研究助理教授陳平。學院博士後研究員周靖波、馮楊洋,博士生儲祥鵬亦對研究做出了重要貢獻。該項目由國家重點研發計劃(檔案編號:2021YFE0206300)、國家自然科學基金(檔案編號:82030094)、澳門大學(檔案編號:CPG2023-00031-FHS和MYRG-GRG2023-00029-FHS-UMDF)和澳門特別行政區科學技術發展基金(檔案編號:0009/2022/AKP、0004-2021-AKP、0007/2021/AKP、0092/2020/AMJ、0054/2023/RIA1和0082/2022/A)支持。全文可瀏覽https://www.sciencedirect.com/science/article/pii/S2452199X24003955。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/campus-news/detail/61430/
UM research team establishes biobank of living tumour tissues to support personalised treatment and research
A research team led by Chuxia Deng, chair professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), has established a biobank of living tumour tissues in collaboration with Kiang Wu Hospital. Standardised cryopreservation technique is used to freeze tumour tissue specimens obtained during early surgical procedures. In cases where tumour samples are unavailable during recurrence or metastasis, the frozen tissues can be revitalised for organoid culture, providing support for potential personalised treatment options for cancer patients or further cancer research. The research has been published in the international journal Bioactive Materials.
A biobank involves the collection and storage of substantial quantities of biological specimens, along with associated personal data such as medical records, family history, lifestyle information, and genetic data. In recent years, there has been a notable increase in the number of large-scale biobanks being established, including those for tumour tissues and other samples. These biobanks typically store specimens under cryogenic conditions using ultra-low temperature freezers or liquid nitrogen. Unfortunately, these storage methods often kill the cells. The specimens can then only be used to extract proteins, DNA, or RNA, and cannot be used to cultivate live cells. This significantly limits their overall value and potential applications.
To address this challenge, the UM research team proposed the novel concept of establishing a biobank of living tumour tissues. They developed a standardised technique in which the tissues are gradually cooled and transferred to liquid nitrogen for long-term preservation. This technique redefines tissue biobanking, as it enables the cultivation of patient-derived tumour organoids (PDOs) from cryopreserved tissues, while supporting air-liquid interface (ALI) cultures and preserving the original tumour immune microenvironment. This technique successfully maintains tissue viability, and holds great promise for advancing personalised precision cancer treatment and research.
In the study, the research team tested 42 pairs of frozen human tumour specimens. Tissue samples from four different cancer types—breast cancer, colorectal cancer, lung cancer, and kidney cancer—were used to establish PDOs. The results showed that organoids could be successfully cultivated in 40 out of 42 cases, achieving a success rate of 95.2%. Notably, the tumour samples could still be used to establish organoid models even after nearly four years of cryopreservation. The study also demonstrated the applicability of this technique for effectively cryopreserving small specimens, including biopsy tissues.
This living tissue cryopreservation technique is simple and easy to operate. It enables various tumour specimens to be frozen long term while maintaining the viability of the cells within the tumour tissues. Organoids derived from these tissues can be routinely frozen and passaged, enabling high-throughput screening of drug sensitivity and identification of potentially effective drugs to combat drug-resistant tumours. Moreover, cryopreserved tumour tissues retain the original tumour microenvironment, making them suitable for ALI cultivation and evaluation of anti-tumour drug responses, including immune checkpoint inhibitors. The study expands the concept of biobanking and lays the foundation for establishing living tissue biobanks, providing substantial support for precision cancer treatment and translational medical research.
Prof Deng is the corresponding author of the study, and Chen Ping, research assistant professor in FHS, is the first author. FHS postdoctoral researchers Zhou Jingbo and Feng Yangyang and FHS doctoral student Chu Xiangpeng also made significant contributions to the study. The project was funded by the National Key R&D Program of China (File No.: 2021YFE0206300), the National Natural Science Foundation of China (File No.: 82030094), UM (File No.: CPG2023-00031-FHS and MYRG-GRG2023-00029-FHS-UMDF), and the Science and Technology Development Fund of the Macao SAR (File No.: 0009/2022/AKP, 0004-2021-AKP, 0007/2021/AKP, 0092/2020/AMJ, 0054/2023/RIA1, and 0082/2022/A). The full version of the research article is available at: https://www.sciencedirect.com/science/article/pii/S2452199X24003955.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/campus-news/detail/61430/