News Express: UM research team reveals new mechanism in regulation of mitophagy
新聞快訊:澳大團隊揭示調控線粒體自噬新機制
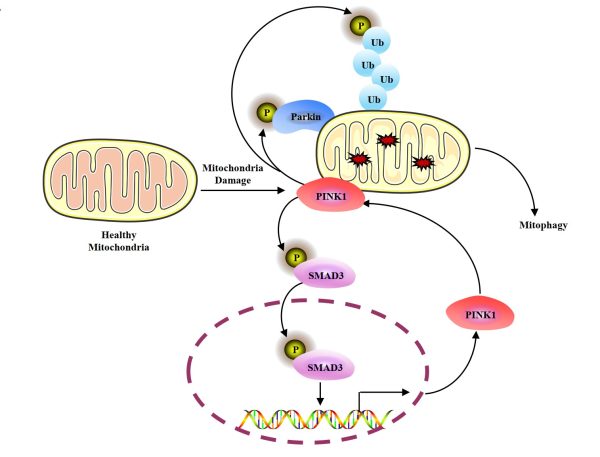
PINK1與SMAD3之間的相互正回饋調控機制是促進線粒體自噬的重要機理
The positive feedback loop between SMAD3 and PINK1 as an important regulatory mechanism in promoting mitophagy
澳大團隊揭示調控線粒體自噬新機制
澳門大學健康科學學院講座教授沈漢明帶領的研究團隊揭示了TGFβ-SMAD信號通路中的關鍵效應因子SMAD3調控PINK1轉錄方面的新機制,對闡明PINK1在帕金森病等神經退行性疾病中的病理功能具有重要的理論價值。相關研究成果已刊登於國際知名期刊《細胞發現》。
線粒體自噬是一種選擇性自噬,作為線粒體品質控制的核心機制,通過自噬-溶酶體途徑選擇性清除受損線粒體,從而維持線粒體穩態。PINK1-Parkin通路是線粒體自噬重要的調控分子機制,當線粒體受損時,PINK1蛋白穩定在線粒體外膜並通過自磷酸化啟動,進而通過磷酸化泛素(Ub)和招募E3泛素連接酶Parkin形成正回饋環路,持續放大線粒體自噬信號。近年來關於PINK1-Parkin下游的調控機制有較為深入的研究,而對於線粒體損傷情況下PINK1自身的調控尤其是其轉錄調控仍未完全闡明。
過往研究表明,線粒體損傷誘導的PINK1穩定主要依賴於其無法被線粒體內膜穿梭作用攝入,從而逃避MMP和PARL蛋白酶的切割作用,最終導致PINK1在線粒體外膜的累積。這一機制被認為是線粒體損傷條件下PINK1累積的主要原因。沈漢明研究團隊發現PINK1在線粒體損傷後的轉錄調控也是PINK1累積的關鍵原因,並通過生物資訊學分析將SMAD3識別為PINK1的潛在轉錄因子。進一步的實驗驗證表明,線粒體去極化能夠顯著促進SMAD3的磷酸化修飾及其核轉位,從而介導PINK1轉錄水準的上調,該調控過程獨立於經典TGFβ信號通路中的關鍵組分(如TGFβ-R1、SMAD2或SMAD4)。進一步研究發現,線粒體去極化誘導的PINK1啟動還參與其自身的轉錄調控:PINK1作為SMAD3的蛋白激酶,通過磷酸化修飾啟動SMAD3,從而促進PINK1的轉錄表達,形成一個正回饋環路,加速線粒體自噬的進程。
線粒體自噬是一種關鍵的細胞存活機制,通過清除受損線粒體來避免由線粒體損傷引發的細胞死亡。該研究揭示,SMAD3通過啟動PINK1的轉錄構建了一個抗凋亡信號通路,為細胞在應對線粒體損傷時提供了重要的生存機制。該研究揭示了SMAD3在PINK1轉錄調控中的新功能,發現了PINK1與SMAD3之間的相互正回饋調控機制,拓展了對SMAD3非經典生物學功能的認識,並闡明其在線粒體品質控制和細胞命運調控中的新功能,這種相互調控機制提高了線粒體自噬的效率。該研究為深入理解線粒體自噬的精細調控機制提供了新的研究視角,同時對闡明PINK1在帕金森病等神經退行性疾病中的病理功能具有重要的理論價值。
該研究的通訊作者為沈漢明,第一作者為澳大健康科學學院博士後唐名珠。澳大健康科學學院的博士生容達德、高向征和唐海梅,生物成像及幹細胞核心實驗中心,基因組學、生物信息學及單細胞分析核心實驗中心成員亦對研究做出重要的貢獻。該項目由澳門特別行政區科學技術發展基金(檔案編號:0078/2020/A2、0031/2021/A1、0081/2022/AMJ、0004/2021/AKP)和澳門大學(檔案編號:CPG2023-00032-FHS、MYRG2020-00022-FHS)資助。全文可瀏覽:https://www.nature.com/articles/s41421-025-00774-4。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/campus-news/detail/61508/
UM research team reveals new mechanism in regulation of mitophagy
A research team led by Shen Hanming, chair professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), has made a breakthrough in clarifying the mechanism by which SMAD Family Member 3 (SMAD3), a key effector in the transforming growth factor beta (TGFβ)-SMAD signalling pathway, regulates PTEN-induced kinase-1 (PINK1) transcription. This study has important theoretical value in elucidating the pathological function of PINK1 in neurodegenerative diseases such as Parkinson’s disease. The findings have been published in the internationally renowned journal Cell Discovery.
Mitophagy is a selective autophagy. As the core mechanism of mitochondrial quality control, it selectively removes damaged mitochondria through the autophagy-lysosome pathway to maintain mitochondrial homeostasis. The PINK1-Parkin pathway is an important regulatory mechanism of mitophagy. When mitochondria are damaged, the PINK1 protein becomes stabilised in the mitochondrial outer membrane and is activated by autophosphorylation to form a positive feedback loop by phosphorylating ubiquitin (Ub) and E3 ubiquitin ligase Parkin for continuously amplifying mitophagy signals. In recent years, there have been in-depth studies on the regulatory mechanism of PINK1-Parkin downstream, but the regulation of PINK1 itself under mitochondrial damage, especially for its transcriptional regulation, remains unclear.
Previous studies have shown that PINK1 stability induced by mitochondrial damage mainly depends on its inability to import into mitochondrial inner membrane, thereby escaping the cleavage by MMP and PARL proteases, ultimately accumulating in mitochondrial outer membrane. This mechanism is considered to be the main reason for the accumulation of PINK1 under mitochondrial damage conditions. Prof Shen’s research team found that the transcriptional regulation of PINK1 after mitochondrial damage is also a key reason for the accumulation of PINK1. They identified SMAD3 as a potential transcription factor for PINK1 through bioinformatics analysis. Further experimental verification showed that mitochondrial depolarisation can significantly promote the phosphorylation and nuclear translocation of SMAD3, thereby upregulating the transcription levels of PINK1. This regulatory process is independent of the key components in the classical TGFβ signalling pathway (such as TGFβ-R1, SMAD2, or SMAD4). Further studies have found that PINK1 activation induced by mitochondrial depolarisation is also involved in its own transcriptional regulation: PINK1, as a protein kinase of SMAD3, activates SMAD3 through phosphorylation, thereby promoting the transcriptional expression of PINK1, forming a positive feedback loop and accelerating the process of mitophagy.
Mitophagy is a key cell survival mechanism that prevents cell death caused by mitochondrial damage by clearing damaged mitochondria. The study revealed that SMAD3 establishes an anti-apoptotic signalling pathway by activating the transcription of PINK1, providing an important survival mechanism for cells in response to mitochondrial damage. The study also discovered a new function of SMAD3 in the transcriptional regulation of PINK1, revealed the mutual positive feedback regulatory mechanism between PINK1 and SMAD3, expanded the understanding of the non-classical biological functions of SMAD3, and clarified its new functions in mitochondrial quality control and cell fate regulation. This mutual regulatory mechanism improves the efficiency of mitophagy. The study provides a new research perspective for a deeper understanding of the fine regulatory mechanism of mitophagy and shows an important theoretical value for clarifying the pathological function of PINK1 in neurodegenerative diseases such as Parkinson’s disease.
The corresponding author of the study is Prof Shen. The first author is Tang Mingzhu, a postdoctoral fellow in FHS. Significant contributions were also made by FHS doctoral students Rong Dade, Gao Xiangzheng, and Tang Haimei, as well as members of the Biological Imaging and Stem Cell Core, and the Genomics, Bioinformatics and Single Cell Analysis Core in FHS. The research project was funded by the Science and Technology Development Fund of the Macao SAR (File No.: 0078/2020/A2, 0031/2021/A1, 0081/2022/AMJ, 0004/2021/AKP), and UM (File No.: CPG2023-00032-FHS, MYRG2020-00022-FHS). The full version of the research article is available at: https://www.nature.com/articles/s41421-025-00774-4.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/campus-news/detail/61508/