News Express: UM research reveals combination therapy enhances proteasome inhibitor-based immunotherapy for solid tumour cells
新聞快訊:澳大研究揭示蛋白酶體抑制劑聯合療法可增強腫瘤療效
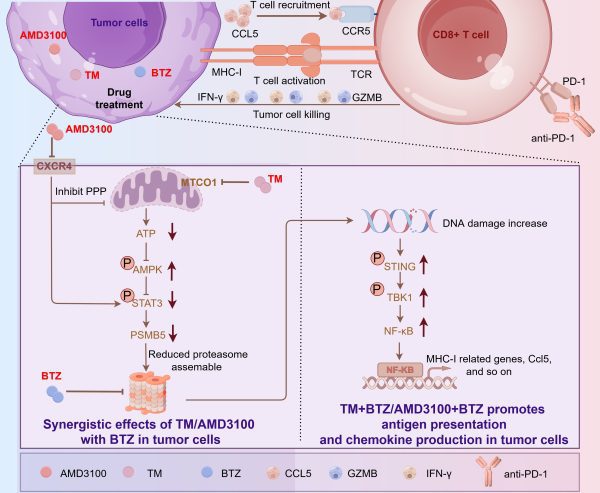
TM及Plerixafor增敏硼替佐米治療效果的機制及藥物組合誘導抗腫瘤免疫活化的機制
The mechanisms by which TM and plerixafor sensitise bortezomib’s therapeutic effect, and the drug combination induces the activation of anti-tumour immunity
澳大研究揭示蛋白酶體抑制劑聯合療法可增強腫瘤療效
澳門大學健康科學學院講座教授鄧初夏的研究團隊在腫瘤研究取得重大進展。該研究利用藥物聯合篩選的方式,發現了提高蛋白酶體抑制劑治療實體腫瘤的策略,並揭示了該藥物組合有效提高免疫檢查點抑制劑對乳腺癌的治療效果,為廣大乳腺癌病患帶來新希望。相關研究成果在生物醫學領域備受矚目,並已發表於國際知名期刊《細胞報告醫學》(Cell Reports Medicine)。
蛋白酶體抑制劑臨床上廣泛應用於多發骨髓瘤等液體腫瘤的治療。澳大研究團隊在過去研究乳腺癌順鉑耐藥機制的工作中,揭示了耐藥細胞通過提升自身蛋白酶體活力,清除化療藥物損傷的蛋白質,進而逃避化療藥物介導的細胞死亡,並發現聯用蛋白酶體抑制劑可以有效逆轉腫瘤多藥耐藥。然而,對於實體腫瘤細胞,儘管在體外展現出對蛋白酶體抑制劑極強的敏感性,但在體內該類藥物並不能有效抑制實體腫瘤的生長。
為解決這一難題,研究團隊設計了藥物聯合篩選的策略,以篩選得到能夠增敏蛋白酶體抑制劑——硼替佐米殺傷腫瘤細胞效果的藥物。篩選發現,兩種已被美國食品藥品監督管理局(FDA)批准的藥物——四硫代鉬酸銨(Ammonium tetrathiomolybdate)和普樂沙福(Plerixafor),可以通過調控AMPK/STAT3/PSMB5信號軸增強乳腺癌細胞對硼替佐米的敏感性。另外,研究團隊還在乳腺癌小鼠模型中發現該藥物組合以CD8+ T細胞依賴的方式發揮抗腫瘤作用,具體的作用機制如下:(1)藥物組合誘導腫瘤細胞產生並分泌CCL5以招募CD8+ T細胞至腫瘤部位;(2)藥物組合啟動腫瘤細胞抗原處理及提呈通路以活化CD8+ T細胞;(3)活化後的CD8+ T細胞負責殺傷腫瘤細胞並放大抗腫瘤免疫效應。該研究結果極具臨床應用潛力,可為未來蛋白酶體抑制劑治療實體腫瘤提供新的藥物組合。
該研究的通訊作者為鄧初夏,第一作者為澳大健康科學學院博士生唐東洋。澳大健康科學學院博士生林詩琪、褚祥鵬、李玲、何林、喬雲鋒,博士後研究員雷海鵬、周靖波,研究助理教授邵方元、孫恆及客席副教授徐曉玲亦對該研究作出貢獻。澳大健康科學學院動物研究核心實驗中心、生物成像及幹細胞核心實驗中心的成員亦對該研究作出重要貢獻。該項目由國家自然科學基金(檔案編號:82030094)、澳門特別行政區科學技術發展基金(檔案編號:0009/2022/AKP、0004/2021/AKP、0007/2021/AKP、0092/2020/AMJ、0054/2023/RIA1)和澳門大學(檔案編號:CPG2023-00031-FHS、MYRG2020-00076-FHS、MYRG2022-00181-FHS、MYRG-GRG2023-00029-FHS)資助。全文可瀏覽:https://www.cell.com/cell-reports-medicine/fulltext/S2666-3791(25)00284-8。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/press-release/detail/61687/
UM research reveals combination therapy enhances proteasome inhibitor-based immunotherapy for solid tumour cells
A research team led by Chuxia Deng, chair professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), has made a breakthrough in cancer research. The study used drug combination screening to identify a novel strategy that enhances the efficacy of proteasome inhibitors against solid tumours. Furthermore, the findings demonstrate that this drug combination can effectively improve the therapeutic effect of immune checkpoint inhibitors against breast cancer, offering new hope for patients. The research has garnered significant attention in the biomedical field and has been published in the prestigious international journal Cell Reports Medicine.
Proteasome inhibitors are widely used in clinical practice to treat liquid tumours, such as multiple myeloma. Previous research by the UM research team on cisplatin resistance mechanisms in breast cancer showed that resistant cells evade chemotherapy-induced cell death by increasing their own proteasome activity to clear proteins damaged by the drugs. The team also discovered that combining proteasome inhibitors could effectively reverse multidrug resistance in tumours. However, although solid tumour cells are highly sensitive to proteasome inhibitors in vitro, these drugs are ineffective at inhibiting solid tumour growth in vivo.
To address this challenge, the research team developed a drug combination screening strategy to identify agents that could enhance the tumour-killing effect of bortezomib, a proteasome inhibitor. The screening identified two FDA-approved drugs, ammonium tetrathiomolybdate (TM) and plerixafor, which can sensitise breast cancer cells to bortezomib by modulating the AMPK/STAT3/PSMB5 signalling axis. Moreover, in breast cancer mouse models, the drug combination exhibited anti-tumour effects in a CD8+ T cell-dependent manner through the following mechanisms: 1) The drug combination induces tumour cells to produce and secrete CCL5, recruiting CD8+ T cells to the tumour site; 2) The drug combination activates the antigen processing and presentation pathway in tumour cells, leading to the activation of CD8+ T cells; and 3) The activated CD8+ T cells kill tumour cells and boost the anti-tumour immune response. This study holds significant clinical potential and provides a novel drug combination strategy for proteasome inhibitor-based therapy against solid tumours.
Prof Deng is the corresponding author of the study, and Tang Dongyang, a PhD student in FHS, is the first author. The following FHS members also contributed to the study: PhD students Lin Shiqi, Chu Xiangpeng, Li Ling, He Lin, and Qiao Yunfeng; postdoctoral fellows Josh Lei Haipeng and Zhou Jingbo; Research Assistant Professors Shao Fangyuan and Sun Heng; and Adjunct Associate Professor Xu Xiaoling. Members of the Animal Research Core and the Biological Imaging and Stem Cell Core in FHS also made significant contributions to the study. The research project was funded by the National Natural Science Foundation of China (File No.:82030094), the Science and Technology Development Fund of the Macao SAR (File No.: 0009/2022/AKP, 0004/2021/AKP, 0007/2021/AKP, 0092/2020/AMJ, and 0054/2023/RIA1), and the University of Macau (File No.: CPG2023-00031-FHS, MYRG2020-00076-FHS, MYRG2022-00181-FHS and MYRG-GRG2023-00029-FHS). The full version of the research article is available at https://www.cell.com/cell-reports-medicine/fulltext/S2666-3791(25)00284-8.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/press-release/detail/61687/