News Express: UM research team develops new method to accelerate cell therapy
新聞快訊:澳大研究為細胞治療關鍵環節提速
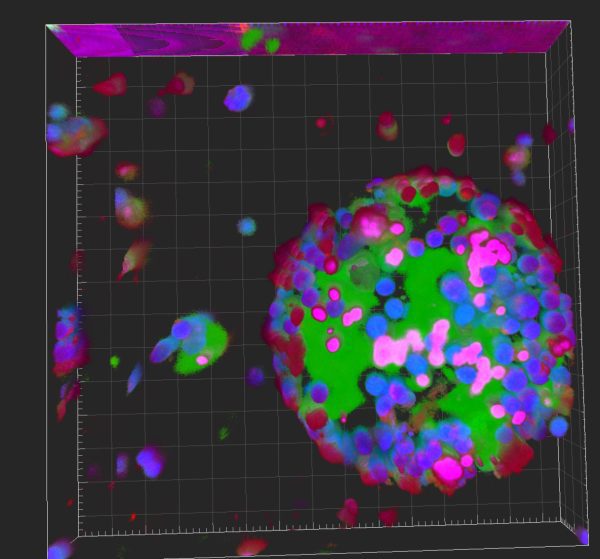
澳大研究團隊發明新型雙水相系統用於大量培養和快速激活治療性細胞
The UM research team develops a novel aqueous two-phase system for large-scale cultivation and rapid activation of therapeutic cells
澳大研究為細胞治療關鍵環節提速
由澳門大學中華醫藥研究院、中藥機制與質量全國重點實驗室教授王春明帶領的研究團隊,開發了一種基於物理化學“相分離”原理的全新工程化細胞培養系統,可顯著縮短免疫細胞活化時間。該研究已發表於國際一流期刊Cell子刊Cell Biomaterials,並與粵港澳大灣區的臨床研究中心開展轉化合作。
細胞治療為多種疾病的治療與組織修復再生提供了新策略。然而,患者來源細胞的活化過程耗時較長,成為臨床轉化應用的關鍵瓶頸。澳大研究團隊最初從細胞內生物大分子凝聚體可促進生物化學反應這一基本科學原理受到啟發,嘗試在細胞表面構建一種加速激活體系。該研究使用了兩種具有良好生物相容性的大分子——聚乙烯醇(PEG)和右旋糖酐(DEX)——構建了一套雙水相系統(ATPS),通過選擇性地將細胞與激活因子共分配,使激活因子以超出常規的濃度富集於細胞分配相,從而快速誘導細胞至所需表型。該研究選取代表巨噬細胞和T細胞的兩類模式細胞進行測試,發現該系統可縮短細胞激活所需時間高達80%,並可將目標基因的表達水平提高至十倍。有趣的是,這套將細胞置放於兩相分離的工程化系統,可顯著增強細胞內相關信號通路中關鍵蛋白(如IKBKG)的液-液相分離事件,整個誘導活化過程都是基於清晰、可控的調節機制。由於全套系統僅採用的兩種生物材料廉價、安全、長期應用且獲多國藥監機構廣泛批准,預期將有助於系統通過後續的監管審批申請,提高臨床與產業界的合作和應用意願。
該研究的共同第一作者為澳大中華醫藥研究院碩士畢業生李雨微和博士後研究員王安恆。深圳大學副教授Massimiliano Galluzzi、西班牙加泰隆尼亞先進化學研究所博士Jordi Esquena、南京大學生命科學學院教授董磊等亦為研究作出重要貢獻。該研究獲澳門特別行政區科學技術發展基金(檔案編號: 0001/2021/AKP、0024/2023/AFJ、005/2023/SKL)及澳門大學(檔案編號:MYRG-GRG2024-00189-ICMS-UMDF、MYRG-GRG2023-00136-ICMS-UMDF、MYRG-CRG2023-00009-IAPME)資助。全文可瀏覽:https://www.cell.com/cell-biomaterials/fulltext/S3050-5623(25)00168-0。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/press-release/detail/61750/
UM research team develops new method to accelerate cell therapy
A research team led by Wang Chunming, professor in the Institute of Chinese Medical Sciences and the State Key Laboratory of Mechanism and Quality of Chinese Medicine at the University of Macau (UM), has developed a novel engineered cell culture system based on the physicochemical principle of phase separation, which can significantly shorten the time required for activating immune cells. The research has recently been published in Cell Biomaterials, a sibling journal to the prestigious international journal Cell, and has prompted a translational collaboration with a clinical research centre in the Guangdong-Hong Kong-Macao Greater Bay Area.
Cell therapy offers new possibilities for treating various diseases and repairing damaged tissue. However, the lengthy activation process of patient-derived cells remains a critical bottleneck in clinical translation. Inspired by the fundamental physical principle that biomolecules may form condensates intracellularly to accelerate biochemical reactions, the UM research team attempted to create a similar system on the cell surface to accelerate activation. They used two biocompatible polymers—polyethylene glycol (PEG) and dextran (DEX)—to construct an aqueous two-phase system (ATPS). By selectively co-partitioning cells and activating factors, the system enriched activating factors in the cell-partitioned phase at concentrations that exceeded conventional levels, thereby rapidly inducing cells to the desired phenotypes. The research team tested the system on two representative model cell types, macrophages and T cells, and found that it could reduce activation time by up to 80% while increasing target gene expression levels by tenfold. Interestingly, this engineered system, which encapsulates cells in a phase-separation environment, markedly enhanced liquid-liquid phase separation of key proteins (such as IKBKG) in relevant signalling pathways of immune activation, with the entire activation process based on clear and controllable regulatory mechanisms. As the system comprises only two inexpensive, safe, and long-approved biomaterials that are widely endorsed by regulatory agencies in multiple countries, it is expected to facilitate regulatory approval applications and elevate interest from clinical and industrial collaborators for the application.
The co-first authors of the study are Li Yuwei, a master’s graduate from the UM Institute of Chinese Medical Sciences (ICMS), and Wang Anheng, a postdoctoral researcher in ICMS. Associate Professor Massimiliano Galluzzi from Shenzhen University, Dr Jordi Esquena from the Institute of Advanced Chemistry of Catalonia in Spain, and Professor Dong Lei from the School of Life Sciences at Nanjing University, among others, also made substantial contributions to the study. The research was funded by the Science and Technology Development Fund of the Macao SAR (File Nos.: 0001/2021/AKP, 0024/2023/AFJ, 005/2023/SKL) and the University of Macau (File Nos.: MYRG-GRG2024-00189-ICMS-UMDF, MYRG-GRG2023-00136-ICMS-UMDF, MYRG-CRG2023-00009-IAPME). The full version of the research article can be accessed at: https://www.cell.com/cell-biomaterials/fulltext/S3050-5623(25)00168-0.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/press-release/detail/61750/