News Express: UM achieves breakthrough in carbon dot-based precision cancer therapy
新聞快訊:澳大利用碳點精準治療腫瘤取得新突破
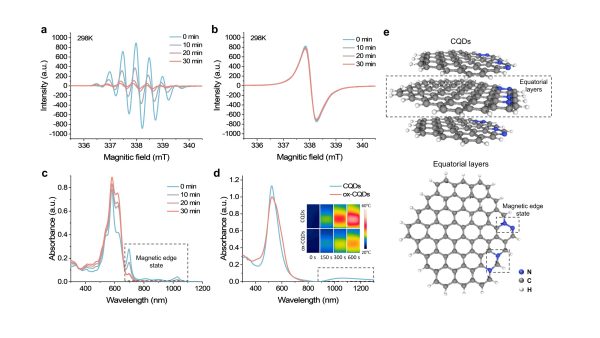
磁邊緣態的結構和電子自旋弛豫行為示意圖
The structure and electron spin relaxation behaviour of magnetic edge states
澳大利用碳點精準治療腫瘤取得新突破
澳門大學應用物理及材料工程研究院講座教授湯子康、特聘教授孫漢東的研究團隊聯合健康科學學院講座教授鄧初夏的研究團隊,開發一種高電子自旋和光學弛豫的碳量子點,該碳點在生物環境中展現出卓越的磁共振成像(MRI)能力及近紅外二區(NIR-II)光驅動的熱電催化性能,因此建立一種MRI引導的NIR-II精準光療新策略。相關研究成果已發表於國際知名期刊《自然通訊》。
碳量子點是納米醫學領域的新興候選藥物,表現出與電子自旋、弛豫和遷移相關的有趣行為,在具備較高的腫瘤靶向能力、有效的腫瘤殺傷效果、治療持續時間短、對身體的副作用小等優點的精準癌症治療中,非金屬碳點因其優異的生物相容性和低細胞毒性而受到青睞,將治療功能與MRI元件相結合是碳點臨床應用中具備潛力的目標。在目前報導的MRI造影劑中,Gd基造影劑的毒性、有機小分子造影劑的低弛豫性和穩定性限制了它們的臨床應用。因此,開發高弛豫性碳基造影劑(如非金屬碳點)至關重要。然而,使用非金屬CQDs在NIR-II視窗中實現光療或MRI挑戰性極高。研究團隊已於過往研究中,通過極化子工程解決碳點在NIR-II吸收係數和光學轉換效率低的難題,相關研究成果亦已發表於國際知名期刊《科學進展》,全文請瀏覽:https://www.science.org/doi/10.1126/sciadv.adn7896。
是次研究則致力引入碳點的磁邊緣態,同時實現MRI和NIR-II癌症治療,顯示出精準癌症治療的潛力。為此,研究團隊通過自由基聚合法合成高順磁性碳量子點,通過電子順磁共振波譜及瞬態吸收光譜,證實其室溫下存在邊緣氮核間快速的電子自旋和光學弛豫。通過調控磁性邊緣態電子弛豫與遷移行為,碳點展現出卓越的MRI T₁成像能力(r₁=270 mM⁻¹s⁻¹)及NIR-II光熱電催化性能。結合蛋白冠介導的腫瘤被動靶向特性,實現了MRI引導的NIR-II精準腫瘤治療。在量子技術背景下,該工作突破碳基材料自旋調控瓶頸,通過結構—配體—環境協同調控,實現超越金屬材料的電子弛豫和遷移特性,為碳基材料在高效自旋平均化、超精細分裂及零場分裂的研究提供新範式,推動納米醫學與自旋電子學發展。
該研究通訊作者為湯子康、鄧初夏、孫漢東和澳大應用物理及材料工程研究院研究助理張特森,第一作者為張特森。該研究由澳門特別行政區科學技術發展基金(檔案編號:0128/2020/A3、0131/2020/A3、0007/2021/AKP、006/2022/ALC、0139/2022/A3)、澳門大學(檔案編號:CPG2020-00026-IAPME、CPG2021-00034-IAPME、CPG2022-00013-IAPME、CPG2025-00034-IAPME、SRG2023-00025-IAPME、MYRG2020-00164-IAPME)、廣東省科學技術廳(檔案編號:2022A0505030024)、深港澳科技計劃項目(C類)(檔案編號:SGDX20210823103803021)、粵港澳光熱電能源材料與器件聯合實驗室(檔案編號:EF010/IAPME-TZK/2020/GDSTC)資助。全文可瀏覽:https://www.nature.com/articles/s41467-025-60951-7。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/campus-news/detail/61911/
UM achieves breakthrough in carbon dot-based precision cancer therapy
A research team led by Chair Professor Tang Zikang and Distinguished Professor Sun Handong in the Institute of Applied Physics and Materials Engineering (IAPME) at the University of Macau (UM), in collaboration with a research team led by Chair Professor Chuxia Deng in the Faculty of Health Sciences (FHS), has developed novel carbon quantum dots (CQDs) that exhibit high electron spin and optical relaxation. These CQDs demonstrate exceptional magnetic resonance imaging (MRI) capabilities and excellent thermoelectric catalytic performance in the second near-infrared (NIR-II) window. This innovation proposes a novel, precise MRI-guided approach to NIR-II cancer phototherapy. The research has been published in the international journal Nature Communications.
CQDs are emerging as promising candidates in the field of nanomedicine. They exhibit behaviours related to electron spin, relaxation, and migration. Thanks to their excellent biocompatibility and low cytotoxicity, non-metallic CQDs are favoured for precision cancer therapy, which boasts high tumour targeting ability, potent tumour-killing efficacy, short treatment durations, and minimal side effects on the human body. The integration of therapeutic functionalities with MRI components is a promising goal for the clinical application of CQDs. Yet, the toxicity of Gd-based contrast agents (CAs) and the low relaxivity and stability of organic small molecule CAs restrict the clinical applications of current MRI CAs. Therefore, it is imperative to develop high-relaxivity carbon-based CAs, such as non-metallic CQDs. However, achieving phototherapy in the NIR-II window using MRI with non-metallic CQDs is highly challenging. In previous research, the research team addressed the issues of the low NIR-II absorption coefficient and optical conversion efficiency of CQDs through polaron engineering. Their findings were published in the prestigious journal Science Advances. The full version of the article is available at: https://www.science.org/doi/10.1126/sciadv.adn7896.
This study focuses on introducing magnetic edge states into CQDs to enable both MRI and NIR-II cancer treatment, thus demonstrating the potential of CQDs for precision cancer therapy. To this end, the research team synthesised highly paramagnetic CQDs using a bottom-up approach based on free radical polymerisation. Electron paramagnetic resonance spectroscopy and transient absorption spectra revealed the magnetic and optical properties, including the presence of fast spin and optical relaxation times of unpaired electrons between two edge nitrogen nuclei at room temperature. Controlling the electron relaxation and migration behaviour of the magnetic edge states enabled the CQDs to exhibit notable MRI T1 imaging ability (r₁ = 270 mM⁻¹s⁻¹) and NIR-II photo-thermoelectric catalytic properties in an aqueous soluation. Furthermore, combining CQDs with protein macromolecules resulted in the formation of protein coronas, enabling the passive enrichment of the CQDs in tumour tissues. Finally, the team successfully developed a precise MRI-guided approach to NIR-II cancer phototherapy. Through the synergistic control of structure, ligands, and environment, the team achieved electron relaxation and migration properties that surpass those of metallic materials. This provides a new paradigm for studying efficient spin averaging, hyperfine splitting, and zero-field splitting in carbon-based materials, thereby advancing the development of nanomedicine and spintronics.
The corresponding authors of the study are Prof Sun Handong, Prof Tang Zikang, Prof Chuxia Deng, and Zhang Tesen, a research assistant in IAPME. The first author is Tesen Zhang. The research project was funded by the Science and Technology Development Fund of the Macao SAR (File Nos.: 0128/2020/A3, 0131/2020/A3, 0007/2021/AKP, 006/2022/ALC, and 0139/2022/A3), the University of Macau (File Nos.: CPG2020-00026-IAPME, CPG2021-00034-IAPME, CPG2022-00013-IAPME, CPG2025-00034-IAPME, SRG2023-00025-IAPME, and MYRG2020-00164-IAPME), the Department of Science and Technology of Guangdong Province (File No.: 2022A0505030024), the Shenzhen-Hong Kong-Macao Science and Technology Innovation Project (Category C) (File No.: SGDX20210823103803021), and the Guangdong-Hong Kong-Macao Joint Laboratory for Photonic-Thermal-Electrical Energy Materials and Devices (File No.: EF010/IAPME-TZK/2020/GDSTC). The full text of the research article is available at: https://www.nature.com/articles/s41467-025-60951-7.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/campus-news/detail/61911/