News Express: UM research reveals connectome-based markers that predict subtypes of frontotemporal dementia
新聞快訊:澳大研究首揭額顳葉癡呆密碼 新影像標誌促精準診斷
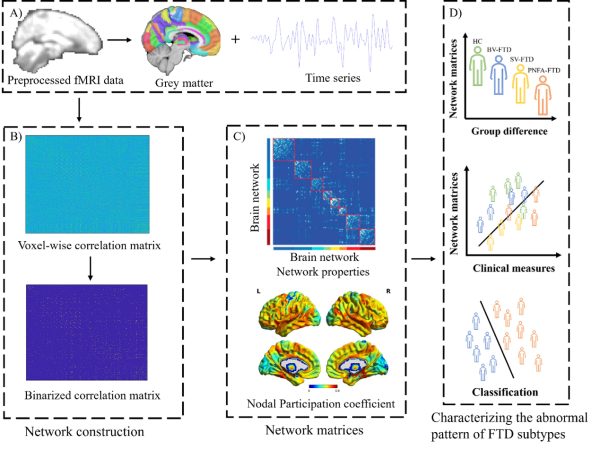
數據分析流程圖
Data analysis flowchart
澳大研究首揭額顳葉癡呆密碼 新影像標誌促精準診斷
由澳門大學認知與腦科學研究中心主任、健康科學學院教授袁振帶領的研究團隊首次揭示了額顳葉癡呆不同亞型在腦功能網絡組織上的顯著差異,並首次提出基於腦連接組的影像學標誌可用於區分及預測額顳葉癡呆的不同類型,為癡呆性疾病的精準診斷提供了新的神經影像學證據。該研究成果已發表於國際頂尖期刊《分子精神病學》(Molecular Psychiatry)。
額顳葉癡呆是最常見的早發性癡呆之一,主要包括三種臨床亞型:行為變異型、語義變異型及進行性非流利性失語型。不同亞型在行為、語言及認知表現上各具特徵,而其背後的腦網絡異常機制仍未被充分揭示。研究團隊利用超過190名受試者的靜息態功能磁共振影像數據,構建高解析度體素級腦連接組,並分析腦模組化組織在不同亞型中的變化特徵,其後進一步結合機器學習模型,實現對各亞型的自動分類與預測。
研究結果顯示,行為變異型與語義變異型患者均表現出皮下結構、默認模式網絡及腹側注意網絡的分離度下降,而行為變異型患者更出現額頂控制網絡的特異性損傷,該網絡與行為控制及執行功能密切相關。相比之下,進行性非流利性失語型患者的網絡分離性較為完整,與其局部語言功能障礙的臨床表現一致。值得注意的是,島葉與眶額皮層的連接改變與患者的情緒及社交行為異常密切相關。
該研究表明,基於腦網絡的神經影像學標誌可為額顳葉癡呆的早期診斷與亞型判別提供重要依據,對推動神經退行性疾病的精準醫學研究具有重要意義。研究成果同時揭示了額顳葉癡呆各亞型之間的共性與特異性神經機制,為未來的干預與治療策略提供了新的方向。
該研究通訊作者為袁振,第一作者為澳大認知與腦科學研究中心博士畢業生、美國馬里蘭大學醫學院助理教授曾星霖。該研究獲澳門大學(檔案編號:MYRG2022-00054-FHS及MYRG-GRG2023-00038-FHS-UMDF)及澳門特別行政區科學技術發展基金(檔案編號:0015/2023/ITP1、0048/2021/AGJ及0020/2019/AMJ)資助。全文可瀏覽:https://www.nature.com/articles/s41380-025-03290-9。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/campus-news/detail/62531/
UM research reveals connectome-based markers that predict subtypes of frontotemporal dementia
A research team led by Yuan Zhen, head of the Centre for Cognitive and Brain Sciences (CCBS) and professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), has unveiled for the first time the significant differences in brain network organisation between different subtypes of frontotemporal dementia (FTD). The team has also identified connectome-based neuroimaging biomarkers that can predict and distinguish between the different FTD subtypes, providing new neuroimaging evidence for the precise diagnosis of this neurodegenerative disorder. The research has been published in the prestigious international journal Molecular Psychiatry.
FTD is one of the most common forms of early-onset dementia and primarily comprises three clinical subtypes—behavioural variant FTD (bvFTD), semantic variant primary progressive aphasia (svPPA), and nonfluent variant primary progressive aphasia (nfvPPA). While each subtype exhibits distinct behavioural, language, and cognitive symptoms, the underlying brain network disruptions remain poorly understood. In this study, the research team analysed resting-state functional MRI data from over 190 participants, including healthy controls and patients with the three major FTD subtypes, to construct high-resolution voxel-wise connectome framework and investigate modular brain organisation across the different subtypes. The team then used machine learning models to classify the FTD subtypes based on these connectome-derived features.
The results revealed that both bvFTD and svPPA groups exhibited decreased modular segregation index (MSI) in the subcortical module, default mode network, and ventral attention network, while patients with bvFTD displayed specific damage in the frontoparietal network, a system crucial for goal-directed behaviour and executive control. By contrast, patients with nfvPPA exhibited relatively preserved modular segregation, which is consistent with their more localised language impairment. It is also notable that network alterations in the insular and orbitofrontal cortex were strongly linked to patients’ emotional and behavioural abnormalities.
This study demonstrates that connectome-based neuroimaging biomarkers can provide important evidence for the early diagnosis and classification of FTD subtypes, which holds significant importance for advancing precision medicine research on neurodegenerative diseases. The findings also shed light on the shared and distinct neural mechanisms underlying the different FTD subtypes, potentially informing future intervention and treatment strategies.
The corresponding author of this study is Prof Yuan. The first author is Zeng Xinglin, doctoral graduate of UM CCBS and assistant professor in the School of Medicine at the University of Maryland. The research project was funded by the University of Macau (File Nos.: MYRG2022-00054-FHS and MYRG-GRG2023-00038-FHS-UMDF) and the Science and Technology Development Fund of the Macao SAR (File Nos.: 0015/2023/ITP1, 0048/2021/AGJ, and 0020/2019/AMJ). The full text of the research article is available at: https://www.nature.com/articles/s41380-025-03290-9.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/campus-news/detail/62531/