News Express: UM unravels ‘double lock’ mechanism of immune checkpoints, offering insight for optimising anti-cancer immunotherapies
新聞快訊:澳大破解免疫檢查點“雙鎖”機制 為優化抗癌免疫療法提供新方向
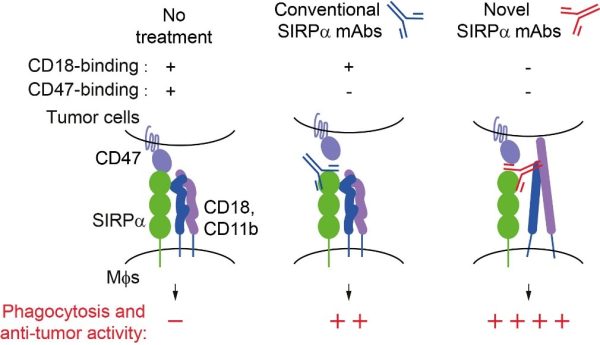
常規與新型SIRPα抗體的吞噬作用及抗腫瘤活性
The phagocytosis and anti-tumour activity of conventional and novel SIRPα antibodies
澳大破解免疫檢查點“雙鎖”機制 為優化抗癌免疫療法提供新方向
澳門大學健康科學學院助理教授唐正海帶領的研究團隊設計了一種可同時阻斷蛋白質SIRPα-CD18及SIRPα-CD47相互作用的雙特異性抗體,能顯著增強巨噬細胞吞噬腫瘤細胞的能力。該研究有望改善癌症治療的新型免疫機制,為開發更精準、毒性更低的免疫療法提供了嶄新靶點,已發表於國際權威期刊《Science Immunology》。
近年,以PD-1和CTLA-4為靶點的單抗療法雖取得顯著成效,但仍有部分患者療效有限或出現復發。為此,科研界開始關注其他免疫細胞,尤其是巨噬細胞在抗腫瘤免疫中的關鍵作用。SIRPα是巨噬細胞表面的典型抑制性受體,當它與腫瘤細胞過度表達的CD47結合時,會向巨噬細胞發出“不要吃我”的信號,阻止其吞噬癌細胞。雖然阻斷 SIRPα-CD47通路的抗體療法能加強免疫反應,但臨床效果仍不穩定,部分患者甚至出現明顯副作用,其潛在原因尚未明確。
澳大團隊揭示了這一現象背後的關鍵原因。研究顯示,即使切斷 SIRPα-CD47之間的反式結合,巨噬細胞的抑制信號仍未完全消失;進一步研究發現,SIRPα能與同一細胞膜上的CD18發生順式結合,抑制CD18的激活與巨噬細胞的吞噬功能。該結合位點與CD47結合位點完全不同,說明SIRPα具有兩種獨立的免疫抑制模式。為驗證該機制並探索新的治療策略,研究團隊設計了一種可同時阻斷SIRPα-CD18及SIRPα-CD47相互作用的雙特異性抗體。實驗結果顯示,該抗體能顯著增強巨噬細胞吞噬腫瘤細胞的能力,並在小鼠模型中有效抑制腫瘤生長,其療效優於單獨阻斷任一通路的抗體。
唐正海指出,免疫檢查點的作用遠比團隊所想的更為複雜,唯有深入理解其在分子層面的多重機制,方能設計出更安全且更高效的治療策略。該研究揭示了免疫檢查點受體的“雙重抑制”模式,為未來抗癌免疫療法的優化提供了嶄新方向。
該研究由唐正海和蒙特利爾臨床研究所教授André veillette帶領的課題組共同完成,獨立第一作者為唐正海,共同通訊作者為唐正海和André veillette。該研究獲澳門特別行政區科學技術發展基金(檔案編號:0090/2024/RIB2)及澳門大學(檔案編號:SRG2024-00027-FHS和UMDF-TISF/2025/002/FHS)資助。全文可瀏覽:https://www.science.org/doi/10.1126/sciimmunol.adv5085。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/press-release/detail/62917/
UM unravels ‘double lock’ mechanism of immune checkpoints, offering insight for optimising anti-cancer immunotherapies
A research team led by Tang Zhenghai, assistant professor in the Faculty of Health Sciences at the University of Macau (UM), has engineered a bispecific antibody capable of simultaneously blocking both the SIRPα–CD18 and SIRPα–CD47 interactions, markedly enhancing macrophage phagocytosis and significantly inhibiting tumour growth. This newly identified immune mechanism could improve cancer treatment and provides a promising target for developing more precise and less toxic immunotherapies. The findings have been published in the prestigious international journal Science Immunology.
While antibodies that block the inhibitory checkpoints PD-1 or CTLA-4 have achieved remarkable success in recent years, many patients still experience limited or short-lived responses. These challenges have prompted researchers to explore other immune cells, particularly macrophages, which play a key role in tumour immunity. SIRPα is a classical inhibitory receptor on macrophages. When bound to CD47, which is frequently overexpressed on tumour cells, SIRPα sends inhibitory signals that block macrophage-mediated phagocytosis and anti-tumour immunity. Although therapies targeting the SIRPα–CD47 axis can enhance anti-tumour immunity, clinical results have been inconsistent, and some patients have experienced side effects, suggesting that other suppressive mechanisms may be involved.
The UM research team has identified a pathway that helps explain this phenomenon. Their study showed that even when the SIRPα–CD47 interaction was disrupted, macrophages still displayed partial inhibition. They found that SIRPα directly cis interacts with CD18 on the cell surface of macrophages, restraining CD18 activation and macrophage phagocytosis. This SIRPα-CD18 interaction occurs on the same cell surface and works together with the known pathway to suppress the immune response. To translate these findings into potential therapy, the team engineered a bispecific antibody capable of simultaneously blocking both the SIRPα–CD18 and SIRPα–CD47 interactions. This antibody markedly enhanced macrophage phagocytosis and significantly inhibited tumour growth in mouse models, demonstrating stronger anti-tumour effects than antibodies blocking either pathway alone.
Tang notes that immune checkpoints are more complex than initially expected. He emphasises that deeper knowledge of their molecular mechanisms is essential for designing safer and more effective cancer therapies. The team’s study reveals a dual-inhibitory mode of immune regulation and offers valuable insight for optimising next-generation immunotherapies.
The study is co-authored by Tang and André Veillette, professor at the Montreal Clinical Research Institute, with Tang as the first author, and Tang and Veillette as corresponding authors. The research was supported by the Science and Technology Development Fund of the Macao SAR (File No: 0090/2024/RIB2) and UM (File No: SRG2024-00027-FHS and UMDF-TISF/2025/002/FHS). The full version of the study is available at: https://www.science.org/doi/10.1126/sciimmunol.adv5085.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/press-release/detail/62917/