News Express: UM research team conducts first comprehensive analysis of gut microbiota-GUS-metabolite axis in colorectal cancer
新聞快訊:澳大團隊首次解析結直腸癌GUSs動態變化全景圖譜
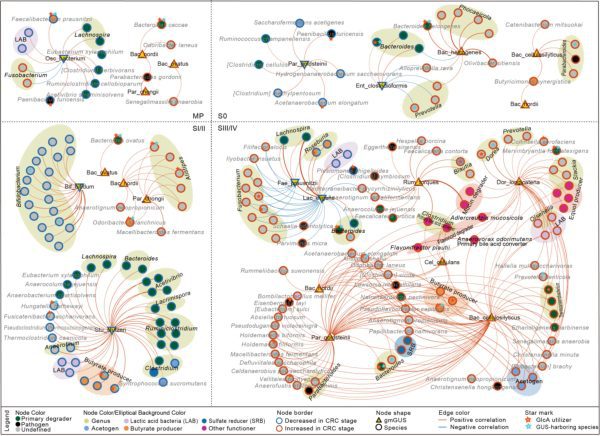
CRC進展中“微生物-GUS-代謝物”軸的動態網絡特徵
Dynamic network characteristics of the microbiota-GUS-metabolite axis during CRC progression
澳大團隊首次解析結直腸癌GUSs動態變化全景圖譜
澳門大學中華醫藥研究院、中藥機制與質量全國重點實驗室教授燕茹帶領的研究團隊,首次系統揭示了腸道微生物β-葡萄糖醛酸酶(GUSs)在結直腸癌(CRC)發生發展過程中的動態變化圖譜,並構建了“微生物-GUS-代謝物”軸在CRC進展中的作用框架。研究成果已發表於國際知名期刊《自然-通訊》。
CRC是全球範圍內的高發惡性腫瘤,且發病呈現年輕化趨勢,其發生發展與腸道菌群失調及相關代謝紊亂密切相關。GUSs是一類廣泛存在於人體腸道微生物中的重要代謝酶,能夠逆轉由宿主肝臟中高表達的尿苷二磷酸葡萄糖醛酸轉移酶(UGTs)所催化的葡萄糖醛酸化“解毒”途徑,參與多種內源性化合物(如膽紅素、甾體激素、膽汁酸等)的代謝穩態調節,以及外源性物質(如藥物、食源性或環境來源的致癌物)的體內處置。
儘管已有研究指出CRC患者糞便中GUS活性顯著高於健康人群,但GUSs在CRC中的整體變化規律及其潛在作用尚未明確。研究團隊基於公共隊列數據,採用一套創新策略,從人體腸道微生物中成功識別出550個GUSs,首次繪製了CRC從腺瘤到晚期階段GUSs的動態失調圖譜,並證實了GUSs在CRC早期診斷及預後預測方面具有良好的潛力。團隊進一步通過構建“微生物-GUS-代謝物”軸,系統闡明該軸在CRC不同階段的擾動特徵,包括有害菌的富集及氨基酸、維生素等代謝通路的顯著紊亂。值得注意的是,通過細胞共培養及轉錄組分析發現,具有階段特異性變化的Bacteroides cellulosilyticus,其GUS可上調腫瘤細胞中RNA轉錄、DNA複製等相關過程,可能潛在地參與癌症進展。
相關發現為未來開發基於GUSs的早期診斷生物標誌物及新型干預靶點奠定了重要的理論與數據基礎。該研究首次全景式解析了CRC發生發展過程中GUS的演變規律及“微生物-GUS-代謝物”軸的擾動模式,為深入理解腸道菌群影響CRC的機制提供了全新視角,也為後續轉化研究奠定了堅實基礎。
該研究通訊作者為燕茹,第一作者為澳大中華醫藥研究院博士生陳俊如,博士生李燕、湯帥和金文煜亦為研究作出重要貢獻。該研究獲澳門特別行政區科學技術發展基金(檔案編號:0091/2021/A2、0098/2019/A2、005/2023/SKL)、深港澳科技計劃專案C類(檔案編號:SGDX20210823103805038)及澳門大學(檔案編號:MYRG-GRG2023-00241-ICMS-UMDF)資助。全文可瀏覽:https://rdcu.be/eR5CM。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/campus-news/detail/63037/
UM research team conducts first comprehensive analysis of gut microbiota-GUS-metabolite axis in colorectal cancer
A research team led by Yan Ru, professor in the Institute of Chinese Medical Sciences (ICMS) and the State Key Laboratory of Mechanism and Quality of Chinese Medicine at the University of Macau (UM), has, for the first time, systematically revealed the dynamic landscape of gut microbial β-glucuronidases (GUSs) during the development of colorectal cancer (CRC) and constructed a functional framework for the ‘microbiota-GUS-metabolite’ axis in CRC progression. The findings have been published in the leading international journal Nature Communications.
CRC is a common malignant cancer worldwide, with its incidence rising among young adults. The onset and progression of the disease are closely linked to gut dysbiosis and related metabolic disturbances. GUSs are an important class of metabolic enzymes widely present in the human gut microbiota. They can reverse the glucuronidation ‘detoxification’ pathway catalysed by highly expressed host hepatic uridine diphosphate-glucuronosyltransferases (UGTs), thereby playing an important role in maintaining the metabolic homeostasis of various biologically important endogenous compounds (such as bilirubin, steroid hormones, and bile acids) and the disposition of exogenous substances (such as drugs and dietary or environmental carcinogens).
Although previous studies have reported significantly higher faecal GUS activity in CRC patients than in healthy individuals, the overall patterns of GUS changes in CRC and their functional roles have remained unclear. By analysing public cohort data using an innovative research strategy, the UM team successfully identified 550 GUSs from the human gut microbiota. They then mapped, for the first time, the dynamic dysregulation of GUSs across disease stages, from adenoma to advanced CRC, demonstrating the promising potential of GUSs for early diagnosis and prognosis prediction in CRC. By constructing the ‘microbiota-GUS-metabolite’ axis, the team systematically elucidated its perturbation characteristics at different CRC stages. This included the enrichment of harmful bacteria and significant disruptions in metabolic pathways such as those for amino acids and vitamins. Notably, cell co-culture experiments and transcriptomic analyses revealed that the GUS from Bacteroides cellulosilyticus, which shows stage-specific changes, can upregulate processes like RNA transcription and DNA replication in tumour cells, potentially contributing to cancer progression.
These findings lay a crucial theoretical and data foundation for the future development of GUS-based early diagnostic biomarkers and novel therapeutic targets. As the first comprehensive analysis of GUS evolution and the perturbation patterns of the ‘microbiota-GUS-metabolite’ axis during CRC development, the study offers a novel perspective for understanding how gut microbiota influence CRC and supports further translational research.
Yan Ru is the corresponding author of the study, with Chen Junru, a doctoral student in ICMS, as the first author. Doctoral students Li Yan, Tang Shuai, and Jin Wenyu also contributed to the research. The study was supported by the Science and Technology Development Fund of the Macao SAR (File Nos: 0091/2021/A2, 0098/2019/A2, and 005/2023/SKL), the Shenzhen-Hong Kong-Macao Science and Technology Programme (Category C) (File No: SGDX20210823103805038), and UM (File No: MYRG-GRG2023-00241-ICMS-UMDF). The full version of the study is available at: https://rdcu.be/eR5CM.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/campus-news/detail/63037/