News Express: UM research reveals mechanisms of NFI family in osteoarthritis, cancer, and development
新聞快訊:澳大研究系統揭示 NFI 家族在骨關節炎、癌症與生長發育中的分子機制
核因子I(NFI)家族轉錄因子的分子機制
Molecular mechanisms of nuclear factor I (NFI) family transcription factors
澳大研究系統揭示 NFI 家族在骨關節炎、癌症與生長發育中的分子機制
澳門大學中華醫藥研究院助理教授宋賀團隊系統闡明核轉錄因子NFI家族在骨關節炎、癌症及生長發育中的作用機制,並基於結構機制探索中藥活性成分以調控該因子功能,為相關疾病治療提供新思路。相關系列研究成果已發表於國際權威期刊《自然—通訊》(Nature Communications)與《核酸研究》(Nucleic Acids Research)。
在結構與代謝調控層面,團隊解析人類NFIA與DNA結合的高解析度晶體與溶液結構,刻畫其對TGGCA基序的序列特異性識別,並定位R116、A123、K125等關鍵殘基與DNA大溝的精確接觸。結合骨關節炎模型研究,結果顯示NFIA調控脂肪酸代謝關鍵酶,其失衡與軟骨細胞代謝紊亂相關,為針對NFIA的結構化干預與後續藥物篩選提供堅實依據。
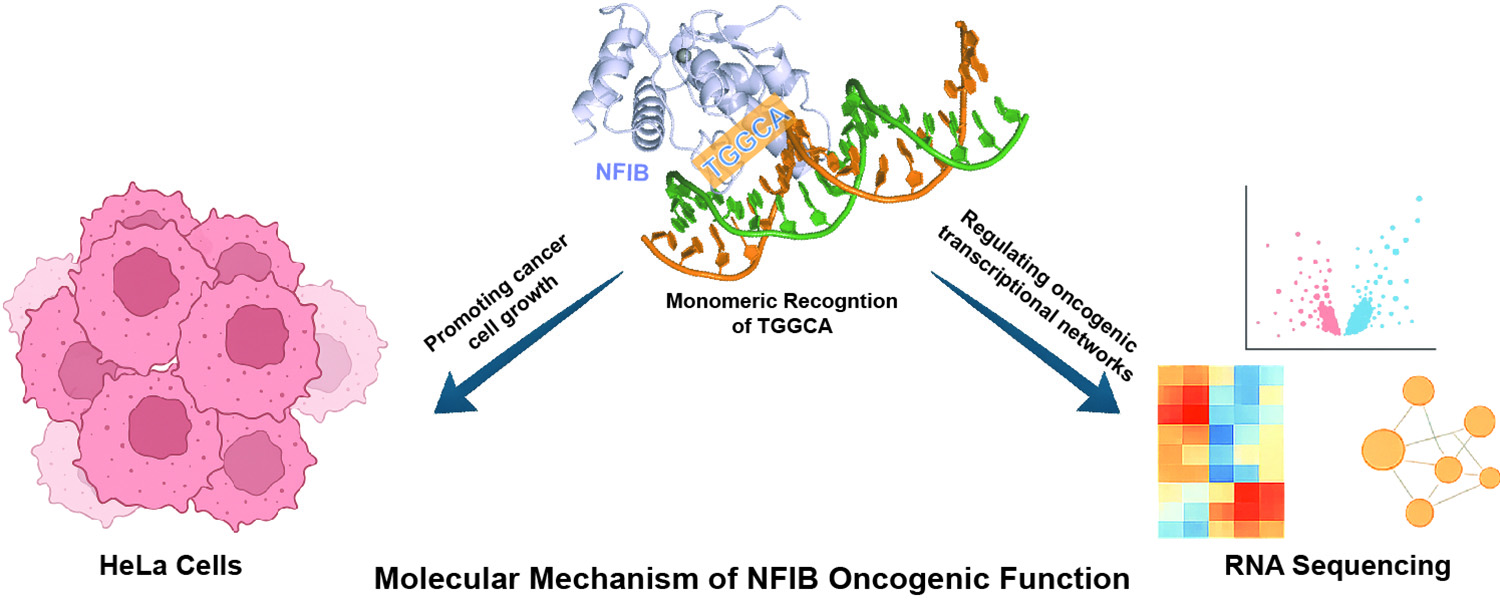
在腫瘤轉錄調控方向,研究獲得NFIB–DNA的原子分辨率複合體結構,並結合 CRISPR敲除與RNA-seq 證據,顯示NFIB在宮頸癌細胞中促進增殖、遷移與侵襲,且調控多個癌症相關基因。結構與突變功能驗證一致表明,NFIB的DNA識別依賴保守殘基的鹼基特異性接觸,為建立精準抑制NFIB的抗癌策略提供分子基礎。
依託上述結構成果,團隊構建了面向轉錄因子結構機制的中藥活性成分篩選體系,打通“從機制到藥效”的技術鏈條,助力從天然產物中發現可調控關鍵轉錄因子的候選分子,推動中醫藥機制化研究與精準治療發展,為骨關節炎與腫瘤等重大疾病提供可轉化的新策略。
該系列研究由澳門大學中華醫藥研究院與美國華盛頓大學聖路易斯分校聯合開展,並與中南大學湘雅醫院、上海中醫藥大學、中國科學院生物物理研究所、湖南大學、蘇州大學、清華大學等多家海內外科研機構合作完成。澳門大學在讀博士朱辤為兩篇論文的第一作者,在讀研究生王月于與陳熙為其中一篇論文的共同第一作者,澳門大學為第一完成單位。研究工作獲澳門特別行政區科學技術發展基金(0068/2023/ITP2;0143/2025/ITP2;0007/2022/AKP;005/2023/SKL)、澳門大學(MYRG-GRG2024-00283-ICMS-UMDF;SRG2023-00054-ICMS)等資助。全文可瀏覽:https://www.nature.com/articles/s41467-025-67641-4(《自然—通訊》);https://academic.oup.com/nar/article/53/22/gkaf1369/8402087(《核酸研究》)。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/campus-news/detail/63137/
UM research reveals mechanisms of NFI family in osteoarthritis, cancer, and development
A research team led by Song He, assistant professor in the Institute of Chinese Medical Sciences (ICMS) at the University of Macau (UM), has systematically revealed the roles of nuclear factor I (NFI) family members in osteoarthritis, cancer, and muscle growth and development. Through structural analysis, the team also explored active compounds from traditional Chinese medicine (TCM) to modulate these transcription factors as potential therapeutic strategies. The findings have been published in the leading international journals Nature Communications and Nucleic Acids Research.
At the structural and metabolic regulatory level, the team determined high-resolution crystal and solution structures of NFIA bound to DNA, defining sequence-specific recognition of the TGGCA motif and mapping key residues that form precise contacts in the DNA major groove. When integrated with osteoarthritis models, the results show that NFIA regulates fatty-acid metabolism enzymes, and that its dysregulation is associated with metabolic imbalance in chondrocytes. These findings provide a structural basis for mechanism-driven intervention and subsequent structure-guided screening.
In the context of cancer transcriptional regulation, the team resolved an atomic-resolution structure of the NFIB–DNA complex and combined this with CRISPR knockout experiments and RNA-sequencing. The results show that NFIB promotes proliferation, migration, and invasion in HeLa cells and modulates the expression of multiple cancer-related genes. Structural analysis and mutational validation further indicate that NFIB’s DNA recognition relies on conserved base-specific contacts, informing the development of targeted anti-cancer strategies.
Building on these structural insights, the team established a high-throughput screening framework for TCM active compounds that target transcription-factor mechanisms. This ‘structure-to-efficacy’ pipeline enables the discovery of natural products capable of modulating key transcription factors, supporting the mechanism-based modernisation of TCM and the development of precision therapeutics. The approach offers promising translational avenues for osteoarthritis, cancer, and other major diseases.
The studies were jointly conducted by UM ICMS and Washington University in St. Louis, in collaboration with Xiangya Hospital of Central South University, Shanghai University of Traditional Chinese Medicine, the Institute of Biophysics at the Chinese Academy of Sciences, Hunan University, Soochow University, and Tsinghua University. UM doctoral student Zhu Ci is the first author of both studies, while UM master’s students Wang Yueyu and Chen Xi are co-first authors of one of the studies. UM is listed as the first affiliated institution in the published articles. The research projects were supported by the Science and Technology Development Fund of the Macao SAR (File Nos: 0068/2023/ITP2, 0143/2025/ITP2, 0007/2022/AKP) and UM (File No: MYRG-GRG2024-00283-ICMS-UMDF, SRG2023-00054-ICMS). The full versions of the research articles are available at: https://www.nature.com/articles/s41467-025-67641-4 (Nature Communications) and https://academic.oup.com/nar/article/53/22/gkaf1369/8402087 (Nucleic Acids Research).
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/campus-news/detail/63137/