News Express: UM research reveals new mechanism underlying synergistic effects between anticancer drugs and immunotherapy
新聞快訊:澳大研究揭示抗癌藥物與免疫療法協同作用新機制
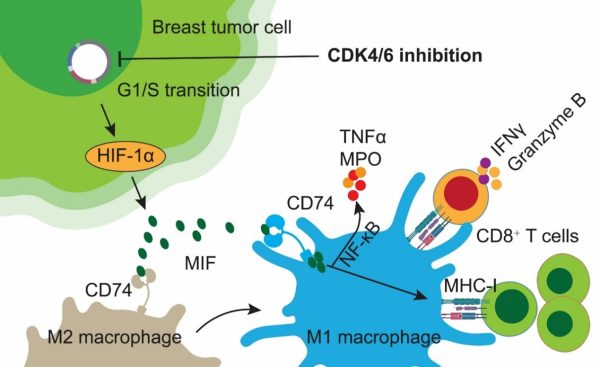
團隊實驗結果機制圖
Mechanism schematic of the study
澳大研究揭示抗癌藥物與免疫療法協同作用新機制
澳門大學健康科學學院講座教授鄧初夏帶領的研究團隊揭示了CDK4/6抑制劑(CDK4/6i)通過調控腫瘤相關巨噬細胞(TAM)亞型,間接啟動CD8+ T細胞抗腫瘤免疫作用,從而顯著提高腫瘤對低劑量PD-1免疫檢查點抑制劑(ICB)療效。研究闡明了腫瘤細胞—巨噬細胞—CD8+ T細胞互作環路的免疫調控新機制,為精準免疫治療帶來新思路,相關成果已發表於知名國際期刊《Advanced Science》。
近年來,CDK4/6i作為腫瘤周期調控類藥物,被廣泛應用於乳腺癌等實體腫瘤治療。該藥物不僅直接誘導腫瘤細胞生長阻滯,還能顯著改善腫瘤微環境(TME)免疫狀態。該項研究通過動物模型、腫瘤組織切片培養、人源腫瘤樣本等多維度證實,CDK4/6i促使腫瘤細胞大量分泌巨噬細胞遷移抑制因數(MIF),進而驅動腫瘤相關巨噬細胞向促免疫的M1型極化。這些M1型巨噬細胞進一步啟動腫瘤內CD8+ T細胞,提升細胞毒性和抗腫瘤效應,從而加強PD-1 ICB免疫治療的回應。
澳大研究團隊還創新性地發現,僅將CDK4/6i訓練獲得的M1型巨噬細胞上清液與低劑量PD-1抗體協同即可增強腫瘤免疫反應,為克服實體腫瘤低應答率、優化免疫治療策略提供了理論依據。該研究同時藉助單細胞轉錄組測序、空間免疫螢光成像等前沿方法,系統展示了CDK4/6i調控免疫微環境的機制和作用網路。
該研究通信作者為鄧初夏,第一作者為澳門大學健康科學學院博士生何林。澳大健康科學學院副教授趙琦和劉子銘,博士後研究員雷海鵬和馮洋楊,博士生彭宇中、唐東洋、褚祥鵬、牟迪、喬雲峰,以及澳門大學生物成像及幹細胞核心實驗中心和動物研究核心實驗中心對該研究作出貢獻;澳門科技大學講座教授譚廣亨研究團隊和科大醫院亦參與研究工作。該研究由澳門特別行政區科學技術發展基金(檔案編號0009/2022/AKP 及0129/2024/RIA2 )資助。全文可瀏覽:https://advanced.onlinelibrary.wiley.com/doi/full/10.1002/advs.202511330。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/campus-news/detail/63169/
UM research reveals new mechanism underlying synergistic effects between anticancer drugs and immunotherapy
A research team led by Chuxia Deng, chair professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), has discovered that CDK4/6 inhibitors (CDK4/6i) regulate tumour-associated macrophage (TAM) subtypes to indirectly activate CD8+ T cell–mediated anti-tumour immunity, thereby significantly enhancing tumour responses to low-dose PD-1 immune checkpoint inhibitors (ICB). This finding reveals a new immunoregulatory mechanism governing the tumour cell–macrophage–CD8+ T cell interaction loop and offers new insights for precision immunotherapy. The study has been published in the leading international journal Advanced Science.
In recent years, CDK4/6 inhibitors have been widely used as cell cycle-regulating drugs for solid tumours such as breast cancer. These agents not only directly induce tumour cell growth arrest but also markedly improve the immune status of the tumour microenvironment (TME). The study confirms this effect across multiple experimental platforms, including animal models, tumour tissue slice cultures, and human tumour samples. Specifically, CDK4/6i prompts tumour cells to secrete large quantities of macrophage migration inhibitory factor (MIF), thereby driving tumour-associated macrophages toward an M1 phenotype that supports immunity. These M1 macrophages further activate intratumoural CD8+ T cells, enhancing cytotoxicity and anti-tumour efficacy, and thereby strengthening responses to PD-1 ICB therapy.
The UM research team also found that conditioned medium from M1 macrophages trained by CDK4/6i, when combined with low-dose PD-1 antibody, can synergistically enhance tumour immune responses. This discovery provides a theoretical basis for overcoming low response rates in solid tumours and for optimising immunotherapy strategies. The study also uses cutting-edge techniques, including single-cell transcriptomics and spatial immunofluorescence imaging, to comprehensively elucidate the mechanisms and networks by which CDK4/6i regulates the immune microenvironment.
Chuxia Deng is the corresponding author of the study, with He Lin, a doctoral student in FHS, as the first author. FHS Associate Professors Zhao Qi and Liu Tzu-Ming; postdoctoral fellows Lei Haipeng and Feng Yangyang; doctoral students Peng Yuzhong, Tang Dongyang, Zhu Xiangpeng, Mou Di, and Qiao Yunfeng; as well as the Biological Imaging and Stem Cell Core and the Animal Research Core at UM also contributed to the study. Collaborative partners included the team led by Tam Kwong Hang, chair professor at the Macau University of Science and Technology (MUST), and the MUST Hospital. The research was supported by the Science and Technology Development Fund of the Macao SAR (File Nos: 0009/2022/AKP, 0129/2024/RIA2). The full version of the research article is available at: https://advanced.onlinelibrary.wiley.com/doi/full/10.1002/advs.202511330.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/campus-news/detail/63169/