News Express: UM research team reveals neural mechanisms in different subtypes of autism
新聞快訊:澳大團隊揭示孤獨症不同亞型神經機制
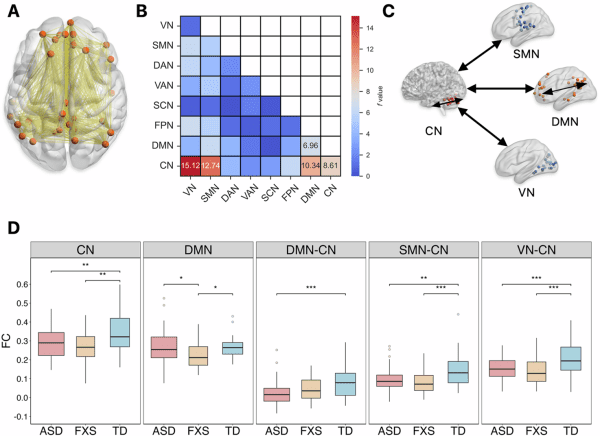
大腦網絡內部和網絡之間平均功能連接存在顯著的組間差異
Comparisons of mean functional connectivity within and between intrinsic brain networks reveal significant group differences
澳大團隊揭示孤獨症不同亞型神經機制
澳門大學認知與腦科學研究中心助理教授李日輝研究團隊與復旦大學附屬兒科醫院教授徐瓊研究團隊合作,在精神病學知名期刊《Molecular Psychiatry》發表兩篇背靠背研究,分別從腦功能連接與腦解剖結構兩個維度,系統揭示了特發性孤獨症(Idiopathic ASD)與脆性X綜合症(FXS,孤獨症的主要單基因遺傳亞型)異同的神經生物學基礎。兩項研究為理解ASD複雜的病理機制提供了突破性見解,並為未來開發針對性干預方案指明方向。
ASD是一種高度異質的神經發育障礙,影響全球約1%至2%的人口。為應對ASD的複雜性,研究團隊選擇了脆性X綜合症—最常見的ASD單基因病因—作為遺傳背景相對清晰的對照模型,以探究兩者行為症狀重疊背後的特異性與共性神經機制。
在第一項研究中,研究團隊利用靜息態功能磁共振成像技術(fMRI),對150名兒童(70名特發性ASD兒童、37名FXS兒童和43名典型發育兒童)的大腦功能連接與網絡拓撲屬性進行分析。研究發現,FXS兒童表現出獨特的大腦功能改變(圖1),他們的默認模式網絡(Default Mode Network)內部連接顯著減弱,該網絡與自我參照認知、記憶處理和情緒調節密切相關。這一特徵清晰地將FXS與ASD及典型發育兒童區分開來。同時,ASD和FXS兒童都表現出小腦網絡內部連接減弱,並且小腦網絡與默認模式網絡、感覺運動網絡、視覺網絡之間的連接也均弱於典型發育組,而FXS兒童則在小腦內部表現出其獨特的節點度數和聚類系數降低。研究還發現了關鍵的腦網路與ASD核心症狀的相關性差異(圖2),在FXS與ASD組中,社會情感和重複刻板行為症狀的嚴重程度與多個腦網絡間的連接強度呈現截然不同的相關性。
第二項研究聚焦於大腦解剖結構的發育軌跡。研究團隊利用T1加權結構磁共振成像數據,對190名兒童(90名特發性ASD兒童、46名FXS兒童和54名典型發育兒童)的灰質體積及其發育軌跡進行分析。研究揭示了FXS在童年早期獨特且顯著的神經解剖學特徵(圖3),與特發性ASD和典型發育組相比,FXS兒童表現出複雜的區域性體積改變:在皮層下結構(尤其是尾狀核)和小腦Crus I區的灰質體積顯著增大,同時,在額葉島蓋皮層、小腦蚓部等關鍵皮層及小腦區域則出現體積減小。這種增生與發育不全並存的模式,構成了FXS清晰的結構性“指紋”。研究進一步發現,這些結構差異並非靜態,而是遵循動態的、年齡依賴的發育軌跡(圖4),FXS的灰質體積差異在2至8歲間的多個狹窄年齡窗口中持續存在。另一關鍵發現在於兩種障礙的生長速率區別(圖5),特發性ASD兒童在全腦及幾乎所有發現異常的腦區都表現出比FXS和典型發育兒童顯著加速的灰質體積增長。相比之下,FXS組的生長軌跡則與典型發育兒童相似,或在一些小腦區域顯著慢於特發性ASD組。結果表明,異常的加速增長是特發性ASD童年早期的一個關鍵特徵,而FXS則遵循不同的發育路徑。
兩項背靠背研究分別從“功能”與“結構”視角,繪製了特發性ASD與FXS在兒童早期大腦發育的精細圖譜。研究一明確了默認模式網絡功能抑制是FXS的神經標誌,而小腦功能障礙是兩者共享的病理基礎;研究二則確立了FXS的特異性腦結構指紋,並發現異常加速增長是ASD的結構性特徵。相關研究成果提示,未來應超越基於行為的診斷,轉向針對ASD不同亞型特定神經發育軌跡的、基於機制的靶向治療。
兩項研究的共同通訊作者為李日輝與徐瓊(研究一和研究二)及復旦大學附屬兒科醫院教授喬中偉(研究一),共同第一作者為澳大博士生馮丹勇和復旦大學附屬兒科醫院醫師李冬蘊。研究獲國家自然科學基金(檔案編號:82171540、82301743)、澳門特別行政區科學技術發展基金(檔案編號:0010/2023/ITP1、0016/2024/RIB1)、澳門大學(檔案編號:SRG2023-00015-ICI、MYRG-GRG2024-00296-ICI、MYRG-CRG2024-00022-ICI)、安徽省自然科學基金(檔案編號:2308085MH255)、中華醫學基金會(CMB)(檔案編號:22-471)、科學技術部外國專家項目(檔案編號:G2022132004L)、復旦大學附屬兒科醫院學科帶頭人發展計劃(檔案編號:EKXDPY202306)及Med+X交叉學科團隊項目資助。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/campus-news/detail/63237/
UM research team reveals neural mechanisms in different subtypes of autism
A research team led by Li Rihui, assistant professor in the Centre for Cognitive and Brain Sciences at the University of Macau (UM), in collaboration with a team led by Xu Qiong, professor in the Children’s Hospital of Fudan University, has published two related studies in Molecular Psychiatry, a leading journal in psychiatry. Together, the two back-to-back publications systematically reveal the distinct and shared neural substrates of idiopathic autism spectrum disorder (ASD) and fragile X syndrome (FXS)—the most common single-gene genetic subtype of ASD—through analyses of brain functional connectivity and anatomical structure. The findings provide important insights into the pathophysiology of these neurodevelopmental disorders and offer guidance for the development of future targeted interventions.
ASD is a highly heterogeneous neurodevelopmental disorder affecting about 1 to 2% of children globally. To address this complexity, the research teams focused on FXS—the most common monogenic cause of ASD—as a genetically more defined model, enabling a clearer investigation of the shared and distinct neural mechanisms underlying the overlapping behavioural features of ASD and FXS.
In the first study, the team used the resting-state functional MRI (fMRI) method to conduct advanced analyses of brain functional connectivity and network topology in 150 children, including 70 children with idiopathic ASD, 37 with FXS, and 43 typically developing (TD) controls. The study found a unique functional alteration in children with FXS (Figure 1). They exhibited significantly weaker intrinsic connectivity within the default mode network (DMN), which is crucial for self-referential cognition, memory, and emotional regulation. This impairment clearly distinguished FXS from both ASD and TD children. At the same time, both ASD and FXS groups showed weaker connections within the cerebellar network (CN), and weaker connections between the CN and other key networks, including the DMN, sensorimotor, and visual network, compared to TD controls. These findings suggest cerebellar dysfunction as a shared neural substrate underlying core behavioural phenotypes. The study also identified critical correlations between brain networks and core symptoms of ASD (Figure 2). The relationship between the severity of core symptoms (social affect and restricted repetitive behaviour) and connectivity between several networks differed for FXS versus ASD.
The second study focused on brain anatomy. The team used T1-weighted structural MRI data from 190 children, including 90 children with idiopathic ASD, 46 with FXS, and 54 TD controls to analyse grey matter volume (GMV) and its developmental trajectories. The findings revealed a pronounced and distinct neuroanatomical signature for FXS in early childhood (Figure 3). Compared with both idiopathic ASD and TD groups, children with FXS exhibited a complex pattern of regional volume alterations: significantly increased GMV in subcortical structures (most notably the caudate nucleus) and cerebellar Crus I, alongside substantial volume reductions in key cortical and cerebellar regions like the frontal insular regions and cerebellar vermis. This combination of overgrowth and undergrowth constitutes a clear structural ‘fingerprint’ for FXS. Importantly, these structural differences were dynamic and age-dependent (Figure 4). The GMV alterations in FXS remained consistent across narrow age ranges between two and eight years. The pivotal discovery was the significant divergence in GMV growth rates between the two disorders. Children with idiopathic ASD demonstrated a pattern of significantly accelerated GMV growth across the whole brain and nearly all regions of interest compared to both FXS and TD groups (Figure 5). In contrast, the FXS group showed growth trajectories either like TD children or significantly slower than the idiopathic ASD group in certain cerebellar regions. These results identify aberrantly accelerated brain growth as a key feature of idiopathic ASD in early childhood, distinguishing it from the developmental path of FXS.
Taken together, the two studies provide a detailed map of early brain development in ASD and FXS from both functional and structural perspectives. The first study identifies DMN hypoconnectivity as a neural signature of FXS and highlights cerebellar dysfunction as a shared pathological foundation, while the second study delineates a specific structural fingerprint for FXS and reveals aberrantly accelerated brain growth as a characteristic of ASD. Collectively, the findings support a move beyond behaviour-based diagnostic frameworks towards mechanism-driven, targeted interventions tailored to the specific neurodevelopmental trajectory of different subtypes of ASD.
Li and Xu are co-corresponding authors of both studies. In the first study, Qiao Zhongwei, professor in the Children’s Hospital of Fudan University also serves as a co-corresponding author. UM doctoral student Feng Danyong and Li Dongyun, a physician at the Children’s Hospital of Fudan University, are co-first authors of both studies. The studies were supported by the National Natural Science Foundation of China (File Nos: 82171540, 82301743), the Science and Technology Development Fund of the Macao SAR (File Nos: 0010/2023/ITP1, 0016/2024/RIB1), UM (File Nos: SRG2023-00015-ICI, MYRG-GRG2024-00296-ICI, MYRG-CRG2024-00022-ICI), the Natural Science Foundation of Anhui Province (File No: 2308085MH255), the China Medical Board (File No: 22-471), the Foreign Expert Program of the Ministry of Science and Technology (File No: G2022132004L), the Academic Leaders Development Program of the Children’s Hospital of Fudan University (File No: EKXDPY202306), and the Med+X Cross-disciplinary Team Project of the Children’s Hospital of Fudan University.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/campus-news/detail/63237/