News Express: UM makes significant progress in novel application strategy for anti-cancer drug
新聞快訊:澳大在抗癌藥物應用取得重大進展
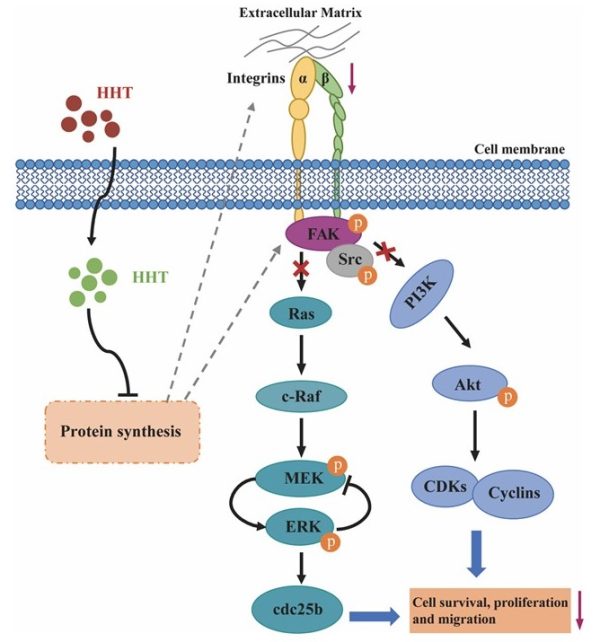
HHT通過抑制integrin α5/β1-FAK/Src下調MAPK/Erk和PI3k/Akt信號通路
A schematic illustration of HHT treatment downregulating the MAPK/Erk and PI3k/Akt signaling pathways by inactivating the integrin α5/β1-FAK/Src axis in bladder cancer
澳大在抗癌藥物應用取得重大進展
澳門大學健康科學學院副教授郭珩輝及其研究團隊在抗癌藥物高三尖杉酯鹼(HHT)的嶄新應用策略上取得重大進展。研究團隊闡明HHT抑制膀胱癌生長的機制,反映HHT作為膀胱癌的治療藥物有巨大潛力,可能應用到膀胱癌的聯合治療策略。研究成果已在國際知名期刊《藥理學研究》(Pharmacological Research)上發表。
膀胱癌是全球十大癌症之一,25%的患者首次診斷為浸潤性膀胱癌或轉移性膀胱癌,此類膀胱癌的5年生存率僅為5%。另外,患者一旦被診斷為膀胱癌,面臨的不僅是有限的治療方案,還有複雜且長期的護理。作為復發率最高的癌種,膀胱癌患者生活質量更差,治療更加困難。
免疫檢查點抑制劑和FGFR蛋白酪氨酸激酶抑制劑在膀胱癌的應用為晚期轉移患者提供了機會。然而,大多數患者在所有階段對免疫治療反應低甚至無反應,且復發患者中出現耐藥的概率十分高。目前,膀胱癌患者迫切需要更新現有的藥物或治療方法,但新藥的成功上市面臨著開發周期長、成本高、成功率低等問題。
HHT在中國用於治療惡性血液病已經有40多年的歷史,並於2012年獲得美國食品藥物管理局(FDA)批准上市。許多研究表明,儘管HHT潛在的作用機制尚不明確,但其可以有效抑制多類實體瘤的惡化。郭教授的研究團隊對HHT抑制膀胱癌生長的機制進行了研究,並將HHT與目前臨床上用於膀胱癌治療的藥物進行了比較。研究發現,HHT表現出比順鉑、卡鉑以及阿奇霉素更強的抑制活性。同時,體內外實驗數據表明,HHT能在納摩爾濃度範圍內有效地抑制膀胱癌細胞增殖、集落形成、遷移以及黏附的能力,並且誘導細胞凋亡和細胞周期阻滯。此外,研究團隊還發現HHT可以通過抑制integrin α5/β1-FAK/Src 下調MAPK/Erk和PI3k/Akt信號通路。HHT通過誘導減少細胞與細胞外基質的相互作用以及細胞遷移,從而抑制了腫瘤轉移。
郭珩輝為該研究的通訊作者,其博士生武秋爽為第一作者。澳大中華醫藥研究院副教授張慶文也為研究作出了重要貢獻。 該研究由澳門特別行政區科學技術發展基金(檔案編號: 0010/2021/AFJ, 0027/2022/A1),廣州市政府科技創新發展專項資金項目對外科技合作計劃(檔案編號: 201807010096)和 澳門大學(檔案編號: MYRG2019-00150-ICMS)資助。全文可瀏覽https://doi.org/10.1016/j.phrs.2023.106654。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/campus-news/detail/55464/
UM makes significant progress in novel application strategy for anti-cancer drug
A research team led by Henry Kwok Hang Fai, associate professor in the Faculty of Health Sciences (FHS) of the University of Macau (UM), has made significant progress in a novel application strategy for the anti-cancer drug Homoharringtonine (HHT). The study has clarified the mechanisms underlying HHT activity against bladder cancer growth and shows the enormous potential of HHT as an anti-cancer agent, which could be applied as a combination treatment strategy for bladder cancer. The research results have been published in the internationally renowned journal Pharmacological Research.
Bladder cancer is among the top ten cancers worldwide. 25 per cent of patients were diagnosed with invasive bladder cancer or metastasis disease for the first time, and the five-year survival rate was only 5 per cent. Once the patients are diagnosed with bladder cancer, they will face limited treatment options and a complex, long-term care pathway. Furthermore, bladder cancer has the highest recurrence rate amount all cancer types. For this reason, patients of this cancer have low quality of life and the therapy is usually more complicated than that for other cancer patients.
Applying immune checkpoint inhibitors and FGFR protein tyrosine kinase inhibitors in bladder cancer therapy provides an opportunity to improve outcomes for patients with high-grade metastatic cancer. However, most bladder cancer patients in all stages do not respond to immunotherapy, and drug-resistant problems occur often in patients with recurrence. There is an urgent need for renewal agents or treatments as new options for patients with bladder cancer. For a new drug to enter the market, there is a long development cycle with high costs and low success rates.
HHT has been used for hematologic malignancies for over 40 years in China and was approved by the United States Food and Drug Administration (FDA) in 2012 as an anti-leukemia drug. Many studies have demonstrated that HHT effectively inhibits the development of several types of solid tumours, although the underlying mechanisms of action are unclear. In this study, Prof Kwok’s research team investigated the mechanisms underlying HHT activity against bladder cancer growth. The research team compared HTT with the drugs currently used clinically for bladder cancer treatment. HHT showed more potent inhibitory activity than cisplatin, carboplatin, and doxorubicin. The in vitro and in vivo data demonstrated that HHT inhibited proliferation, colony formation, migration, and cell adhesion of bladder cancer cells and induced apoptosis and cell cycle arrest in the nanomolar concentration range. Furthermore, the research team revealed that HHT treatment could downregulate the MAPK/Erk and PI3k/Akt signaling pathways by inactivating the integrin α5/β1-FAK/Src axis. In addition, HHT-induced activity reduced cell-extracellular matrix (ECM) interactions and cell migration, thus suppressing tumour metastasis progression.
Prof Kwok is the corresponding author of this study and his PhD student Wu Qiushuang is the first author. In addition, Zhang Qingwen, associate professor in UM’s Institute of Chinese Medical Sciences, also made important contributions to this study. The study was supported by the Science and Technology Development Fund, Macao SAR (File no: 0010/2021/AFJ, 0027/2022/A1), the Guangzhou Science and Technology Innovation Funding (File no: 201807010096) and UM (File no: MYRG2019-00150-ICMS). The full version of the research study can be viewed at https://doi.org/10.1016/j.phrs.2023.106654.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/campus-news/detail/55464/