News Express: UM successfully develops TME nanoprobe for tumour therapy
新聞快訊:澳大成功研發TME腫瘤診療探針
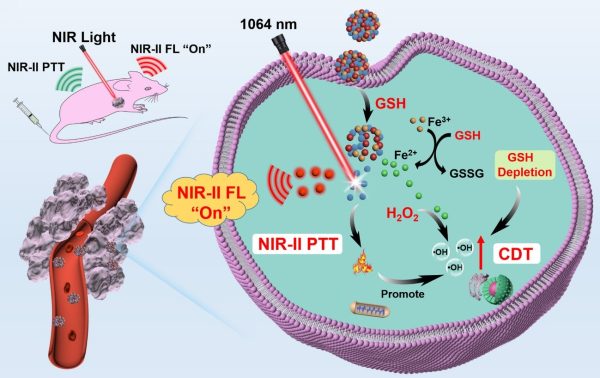
腫瘤特異性NIR-II螢光成像和協同的NIR-II光熱-化學動力學治療的示意圖
A schematic illustration of tumour-specific NIR-II fluorescence imaging and the synergistic NIR-II photothermal-chemodynamic therapy
澳大成功研發TME腫瘤診療探針
澳門大學健康科學學院副教授趙琦的研究團隊和南京郵電大學材料科學與工程學院教授沈清明的研究團隊合作,在腫瘤微環境(TME)可激活的第二近紅外窗口(NIR-II,1000-1700 nm)光學診療納米平台的開發上取得重要進展。研究團隊通過簡單配位驅動的自組裝策略成功製備了TME激活的納米光學診療探針,可應用於靈敏和特異性的腫瘤診斷上,同時產生協同的抗腫瘤治療效果。該研究已發表於國際學術期刊Small。
NIR-II中的光學診療技術,能減少生物樣品的光子吸收和散射,加深組織穿透深度,具有較高的輻射最大允許暴露,因此在腫瘤診療上具有應用潛力,但目前大多數NIR-II探針處於“始終開啟”模式,由於非特異性作用,其在病變和正常組織中均會發出恒定的螢光信號,可能導致較低的腫瘤與正常組織信號比,從而降低腫瘤成像的靈敏度和特異性,甚至出現“假陽性”結果。另外,非特異性的診斷和治療可能在脫靶治療期間對正常組織造成不可逆轉的損傷。
一些激活型NIR-II光學診療探針已被開發,通過響應TME精確區分病變組織與正常組織。儘管它們具有顯著的診斷特異性,但目前開發的激活型NIR-II探針主要受限於複雜的化學合成步驟。此外,為了遞送螢光團,可能需要引入額外的組分,從而因賦形劑而產生副作用。因此,需要開發一種簡便的方法來製備激活型NIR-II光學系統的TME,以提高對腫瘤的診斷特異性和治療效果。
因此,研究團隊基於福斯特共振能量轉移(FRET)原理開發了一種TME激活的光診療納米平台(AFD NPs)。AFD NPs以三價鐵(Fe(III))作為配位節點,通過硫化銀量子點(Ag2S QDs, NIR-II螢光探針)和超小半導體聚合物點(DBZ Pdots,NIR-II螢光淬滅劑)的自組裝製備得到。在正常組織中,由於Ag2S QDs和DBZ Pdots之間的FRET效應,AFD NPs能始終保持“關閉”狀態。然而,AFD NPs的NIR-II螢光信號可被腫瘤組織中過表達的谷胱甘肽(GSH)快速“開啟”,從而顯著增強腫瘤與正常組織(T/NT)的成像信號比,實現腫瘤特異性的診斷。此外,釋放的Pdots和還原產生的Fe(II)離子分別提供NIR-II光熱治療(PTT)和化學動力學治療(CDT)。GSH耗竭和NIR-II PTT效果進一步加劇了CDT介導的對腫瘤的氧化損傷,實現了協同的抗腫瘤治療效果。該研究為開發激活型NIR-II光診療納米探針的TME提供了前景策略。
該研究的通訊作者為趙琦和沈清明,澳大博士後戴葉能為第一作者。此項研究獲澳門特別行政區科學技術發展基金(檔案編號: 0043/2021/A1),國家重點研發計劃(檔案編號:2019YFA0904403)和澳門大學(檔案編號:MYRG2022-00143-FHS)資助。全文可瀏覽:https://onlinelibrary.wiley.com/doi/full/10.1002/smll.202206053。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/campus-news/detail/55521/
UM successfully develops TME nanoprobe for tumour therapy
A research team led by Zhao Qi, associate professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), and a team led by Shen Qingming, professor in the School of Telecommunication and Information Engineering at the Nanjing University of Posts and Telecommunications, have made significant progress in developing a tumour microenvironment (TME) activated phototheranostic nanoplatform in the second near-infrared window (NIR-II, 1000-1700nm). They succeeded in fabricating a TME-activated nanoprobe through a simple coordination-driven self-assembly strategy, which can achieve sensitive and specific tumour diagnosis and the synergistic anti-tumour therapeutic effect. The research results have been published in the international journal Small.
NIR-II phototheranostics have shown great prospects in tumour theranostics due to the depressed photon absorption and scattering by biological samples and can achieve deeper tissue penetration and higher maximum permissible exposure to lasers. However, most current NIR-II probes are in an ‘always on’ mode, which emit invariable fluorescence signals in both lesion and normal tissues owing to their non-specific effects. Such ‘always on’ signal mode can lead to a low tumour-to-normal tissue signal ratio, limited sensitivity and specificity, and even ‘false positive’ results. The non-specific diagnosis and treatments may cause irreversible damage to normal tissues during the off-target theranostic period.
Some activatable NIR-II phototheranostic probes have been fabricated to precisely distinguish lesions from normal tissues in response to TME. Despite excellent diagnostic specificity, current activatable NIR-II probes are mainly limited by complex chemical synthesis steps. In addition, to deliver the fluorophore, it may be necessary to introduce excess components, which might cause some excipient side effects. Therefore, it is highly desirable to develop a facile method to fabricate TME activatable NIR-II phototheranostic systems for improving the diagnosis specificity and therapeutic efficacy toward tumours.
In view of this, the research team developed a TME-activated phototheranostic nanoplatform (AFD NPs) based on the principle of Förster resonance energy transfer (FRET). The AFD NPs are fabricated through self-assembly of Ag2S QDs (NIR-II fluorescence probe) and ultra-small semiconductor polymer dots (DBZ Pdots, NIR-II fluorescence quencher) utilising Fe(III) as coordination nodes. In normal tissues, the AFD NPs maintain themselves in an ‘off’ mode, due to the FRET between Ag2S QDs and DBZ Pdots. However, the NIR-II fluorescence signal of AFD NPs can be rapidly ‘turned on’ by the overexpressed glutathione (GSH) in tumour tissues, resulting in significantly enhanced tumour-to-normal tissue (T/NT) signal ratio for tumour-specific diagnosis. Moreover, the released Pdots and reduced Fe(II) ions provide NIR-II photothermal therapy (PTT) and chemodynamic therapy (CDT), respectively. The GSH depletion and NIR-II PTT effect further aggravate CDT-mediated oxidative damage toward tumours, achieving the synergistic anti-tumour therapeutic effect. The work provides a promising strategy for the development of TME-activated NIR-II phototheranostic nanoprobes.
Prof Zhao and Prof Shen are the corresponding authors of the study, and UM postdoctoral fellow Dai Yeneng is the first author. The project was supported by the Science and Technology Development Fund of Macao SAR (File no: 0043/2021/A1), the National Key R&D Programme of China (File no: 2019YFA0904403), and UM (File no: MYRG2022-00143-FHS). The full version of the research paper can be viewed at https://onlinelibrary.wiley.com/doi/full/10.1002/smll.202206053.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/campus-news/detail/55521/