News Express: UM makes new progress in intelligent diagnosis of Alzheimer’s Disease
新聞快訊:澳大在阿茲海默症的智能診斷研究取得新進展
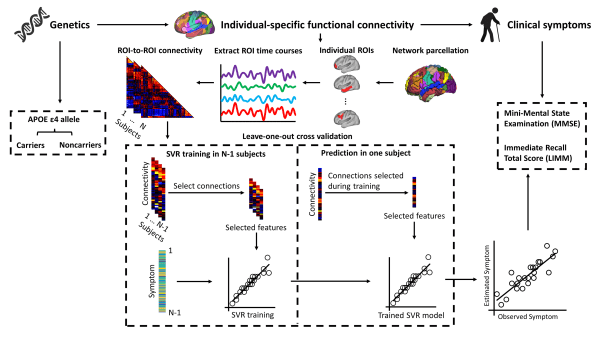
使用個體化腦功能網絡和AI預測APOE ε4等位基因攜帶者與非攜帶的老年人群AD相關的臨床症狀
The use of individual-specific functional connectivity and AI to predict symptoms associated with AD in carriers and non-carriers of the APOE ε4 allele in the elderly population
澳大在阿茲海默症的智能診斷研究取得新進展
澳門大學認知與腦科學研究中心教授袁振的研究團隊在個體化腦功能網絡圖譜預測正常認知老化(CA)、輕度認知功能障礙(MCI)與阿茲海默症(AD)的研究中取得新進展。研究首次結合個體化腦功能網絡和人工智能技術(AI),揭示不同APOE ε4基因型的老年人從CA、MCI 到AD的腦功能網路圖譜差異,從而實現基於個體化的AD智能診斷。研究成果已於Nature旗下期刊Communications Biology刊登。
AD是最常見的神經退行性疾病之一,截至2019年,全球AD及其他痴呆患病人數超過516萬。AD患者通常伴有記憶和執行功能缺陷,進而導致日常生活困難。傳統神經影像學研究依賴群體水平的腦功能圖譜,難以對AD的神經標誌物進行精準診斷。在個體水平上,特別是對於具有不同臨床表現的患者預測從CA、MCI到AD過程的研究仍然匱乏。
研究人員採用235名(120名APOEε4等位基因攜帶者和115名APOE ε4等位基因非攜帶者)來自Alzheimer’s Disease Neuroimaging Initiative(ADNI)數據集中的老年人群神經影像學數據(年齡:60-85歲),並對每個個體構建基於個體水平的腦功能連接網絡。隨後利用基於遞歸特徵選擇的機器學習模型來揭示個體大腦和行爲的關係,並預測不同基因型的AD臨床症狀轉變。研究表明不同APOEε4基因型的老年人群個體化腦功能網絡能夠預測從CA、MCI到AD的臨床亞組,並對AD相關的臨床症狀表現出較高的預測水平。然而傳統基於腦圖譜的功能網絡在幾乎所有情況下都無法有效預測這些臨床症狀。
同時,該研究也表明腦功能區域間的連接比腦功能區域內的連接更能解釋AD症狀特異性嚴重程度,尤其是對具有APOEε4等位基因的老年人群。研究結果強調了在神經退行性疾病研究中考慮皮層功能個體差異的重要性,並可以擴展到健康人群。此外,個體化功能腦網絡模型可以提供個性化治療策略。因此,該研究強調了個體化腦功能網絡和臨床症狀之間的關係,這爲AD的診斷、預後和治療提供臨床效用。
該研究由袁振帶領,澳大認知與腦科學研究中心、健康科學學院博士生華林爲第一作者。該課題由澳門特別行政區科學技術發展基金(0048/2021/AGJ 和0020/2019/AMJ)、澳門特別行政區政府教育基金(CP-UMAC-2020-01)和澳門大學(MYRG2020-00067-FHS和MYRG2019-00082-FHS)聯合資助。全文可瀏覽:https://www.nature.com/articles/s42003-023-04952-6。
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/campus-news/detail/55868/
UM makes new progress in intelligent diagnosis of Alzheimer’s Disease
A team led by Yuan Zhen, professor at the Centre for Cognitive and Brain Sciences (CCBS) of the University of Macau (UM), has made new progress in the study of individual-specific functional connectivity to predict normal cognitive ageing (CA), mild cognitive impairment (MCI), and Alzheimer’s disease (AD). This study is the first to combine individual-specific functional connectivity with artificial intelligence (AI) techniques to reveal differences in functional connectivity between CA, MCI, and AD in elderly people with different APOE ε4 genotypes. The research results have been published in Communications Biology, a journal from Nature.
AD is one of the most common neurodegenerative diseases. As of 2019, over 5.16 million people worldwide were diagnosed with AD or other types of dementia. AD patients usually suffer from significant memory loss and executive function impairments, which lead to decreased quality of life. Traditional neuroimaging investigations relying on population-level functional network atlas have difficulty in identifying brain biomarkers for the accurate diagnosis of AD. At the individual level, studies on predicting the process from CA, MCI, to AD are still insufficient, particularly in patients presenting different clinical phenotypes.
In the study, researchers extracted the neuroimaging data of 235 elderly individuals (120 APOE ε4 carriers and 115 APOE ε4 non-carriers; age: 60-85 years) from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) dataset and constructed individual-specific functional connectivity for each participant. They then utilised recursive feature selection-based machine learning to reveal individual brain-behaviour relationships and predict changes in the clinical symptoms in AD patients with different genotypes. The study found that individual-specific functional connectivity of elderly people with different APOE ε4 genotypes could be used to predict the clinical subgroups from CA, MCI, to AD, and yield moderate-to-strong estimation levels of AD-related clinical symptoms. However, the team failed to detect clinical symptoms in almost all cases with conventional atlas-based functional connectivity.
The study also showed that connections between brain functional networks are more informative than those within the same network in explaining symptom-specific severity of AD, particularly for elderly people with the APOE ε4 allele. The research results highlighted the importance of accounting for individual variation in cortical functional anatomy in neurodegenerative research, which can be extended to the healthy population. In addition, individual-specific functional connectivity models could be used to tailor treatment to specific needs. Thus, the study underlined the relationship between individual-specific functional connectivity and clinical symptoms, which can provide clinical utility for the diagnosis, prognosis, and treatment of AD.
The study was led by Prof Yuan, and the first author of the paper is Hua Lin, PhD student in the CCBS and the Faculty of Health Sciences. The study was supported by the Science and Technology Development Fund, Macao SAR (File no: 0048/2021/AGJ, 0020/2019/AMJ), Macao SAR Government Education Fund (CP-UMAC-2020-01), and UM (MYRG2020-00067-FHS, MYRG2019-00082-FHS). The full version of the paper can be viewed at https://www.nature.com/articles/s42003-023-04952-6.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/campus-news/detail/55868/