News Express: UM develops nanomedicine to enhance breast cancer immunotherapy
新聞快訊:澳大納米藥提升乳腺癌治療成效
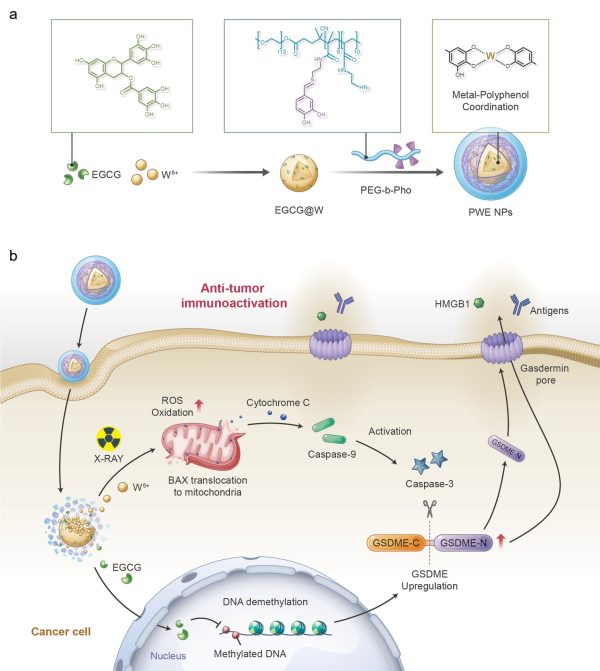
放療藥物(PWE NPs)的合成示意圖及治療策略
A schematic of the synthesis of PWE NPs and therapeutic strategy
澳大納米藥提升乳腺癌治療成效
澳門大學健康科學學院副教授代雲路的研究團隊成功研發新型納米藥物,將傳統放射治療介導的癌細胞凋亡轉換為細胞焦亡,進而更有效地激發系統性抗腫瘤免疫反應,抑制乳腺癌生長、復發及轉移。研究成果已刊登於著名學術期刊《先進功能材料》。
傳統放射療法方式受限於局部病灶的治療,難以有效激發系統性抗腫瘤免疫反應。放射療法主要通過引發細胞凋亡來抑制腫瘤細胞生長,而這種凋亡方式在激發體內的抗腫瘤免疫反應方面可能設置諸多障礙。發生凋亡的癌細胞仍可保持膜的完整性,其內部隱藏的豐富免疫原性損傷相關分子(DAMPs)難以釋放以喚醒周圍的免疫細胞。
研究團隊通過將功能化的天然多酚與金屬離子配位,構建了一種金屬-多酚配位納米藥物PWE,通過表觀遺傳學的方法將放射治療介導的細胞凋亡轉換為細胞焦亡,進而激發系統性抗腫瘤免疫反應。研究結果證明,兩親性PEG-多酚聚合物和DNA甲基轉移酶抑制劑(表沒食子兒茶素,EGCG)能與放療增敏劑(鎢離子,W6+)通過金屬-多酚配位的方法自組裝成核殼結構的放療增敏藥物PWE。EGCG通過抑制DNA甲基化誘導細胞焦亡的關鍵蛋白-GSDME高表達;同時金屬離子W6+在X 射線輻照下產生大量的活性氧自由基,進而通過Bax-Cytochrome c-caspase-9-caspase-3通路激活半胱天冬酶-3(Caspase-3)。激活狀態的Caspase-3可進一步將高表達GSDME 蛋白切割成為GSDME-N蛋白,進而在腫瘤細胞膜上進行打孔。HMGB1和乳酸脫氫酶(LDH)等細胞內容物隨即可通過膜上的孔洞釋放出來,高效激活系統性抗腫瘤免疫反應。在原位乳腺癌小鼠模型中發現,PWE聯合X射線輻照可以通過介導乳腺癌細胞焦亡顯著激發機體內的抗腫瘤免疫響應,抑制乳腺癌生長、復發及轉移。該種療法利用金屬-多酚配位藥物通過表觀遺傳學的方法將傳統放療介導的癌細胞凋亡巧妙地轉變為細胞焦亡,促進了傳統放療的免疫治療效果,為臨床放射治療提供了新的思路。
該研究的通訊作者為代雲路,澳大健康科學學院博士畢業生王國浩和研究助理教授李蓓為共同第一作者,博士後謝麗斯和李傑,博士畢業生桑瑋和張展,博士生田浩、顏潔和李文曦亦對該研究作出重要貢獻。該研究由澳門特別行政區科學技術發展基金(檔案編號:0109/2018/A3、0011/2019/AKP、0113/2019/A2、0103/2021/A和0002/2021/AKP)、澳門大學(檔案編號:MYRG2022-00011-FHS)及深港澳科技計劃項目(C類)(檔案編號:SGDX20201103093600004)資助。全文可瀏覽:https://onlinelibrary.wiley.com/doi/10.1002/adfm.202213425
欲瀏覽官網版可登入以下連結:
https://www.um.edu.mo/zh-hant/news-and-press-releases/campus-news/detail/56189/
UM develops nanomedicine to enhance breast cancer immunotherapy
A research team led by Dai Yunlu, associate professor in the Faculty of Health Sciences (FHS) at the University of Macau (UM), has designed a novel nanomedicine, which can convert traditional radiotherapy-mediated apoptosis into pyroptosis, stimulate systemic anti-tumour immune response, and significantly inhibit breast cancer growth, recurrence, and metastasis. The research results have been published in the renowned journal Advanced Functional Materials.
Radiotherapy, although clinically effective for localised tumours, still cannot radio-functionalise them as a potent immunogenetic centre. This is because radiotherapy inhibits tumour cell growth by inducing apoptosis, such apoptotic nature limits its activating performance on systemic immunosurveillance. Apoptotic cancer cell maintains an intact membrane that conceals abundant potentially immunogenic damage-associated molecular patterns (DAMPs) inside, the releasing of whom, otherwise, could evoke surrounding immune cells remarkably.
By W6+-coordinating natural polyphenols and chemically synthesised polyphenol derivatives, the research team developed a metal-polyphenolic nanocoordinator (PWE), which can epigenetically convert radiotherapy-mediated cell apoptosis to pyroptosis, thereby highly efficiently stimulating a systemic anti-tumour immune response. Specifically, PWE could be constructed via metal-phenolic coordination between poly-phenolic DNA methyltransferase (epigallocatechin-3-gallate, EGCG), high-Z radiosensitiser (W6+), and polyphenol-modified block copolymer. EGCG downregulated DNA hypermethylation of tumour cells and recovered their normal expression of the functional GSDME protein, a key protein of pyroptosis, while noble tungsten ions (W6+) sensitised by X-ray radiation boosted the production of cellular reactive oxygen species (ROS) that initiated the activation of caspase-3 through a potential pathway of Bax-Cytochrome c-caspase-9-caspase-3. The activated caspase-3 could be utilised to cleave GSDME upregulated by an EGCG demethylation, generating GSDME-N for cellular membrane perforation. The formed gasdermin pores enabled intracellular contents of GSDME, HMGB1, and lactate dehydrogenase (LDH) to pass through and release out of the cell, stimulating the immune system and reinvigorating anti-tumour immune responses. Furthermore, in treating 4T1 orthotopic breast tumour-bearing mice with the PWE nanomudulators, the research team identified efficient anti-tumour immune activity, long-lasting immunological memory, and metastatic inhibition. The nanocoordinator developed by metal-polyphenol coordination reshapes and ameliorates tumour immunity in traditional radiotherapy significantly through epigenetic pyroptosis, which may provide new insights into clinical radiotherapy.
The corresponding author of the study is Prof Dai. FHS PhD graduate Wang Guohao and Research Assistant Professor Li Bei are the co-first authors. Postdoctoral fellows Xie Lisi and Li Jie, PhD graduates Sang Wei and Zhang Zhan, PhD students Tian Hao, Yan Jie, and Li Wenxi also made important contributions to the study. The research project was supported by the Science and Technology Development Fund of the Macao SAR (File no: 0109/2018/A3, 0011/2019/AKP, 0113/2019/A2, 0103/2021/A, and 0002/2021/AKP), UM (File no: MYRG2022-00011-FHS), and the Shenzhen-Hong Kong-Macao Science and Technology Innovation Project (Category C) (File no: SGDX20201103093600004). The full version of the research article can be viewed at https://onlinelibrary.wiley.com/doi/10.1002/adfm.202213425.
To read the news on UM’s official website, please visit the following link:
https://www.um.edu.mo/news-and-press-releases/campus-news/detail/56189/